Fig. 2.
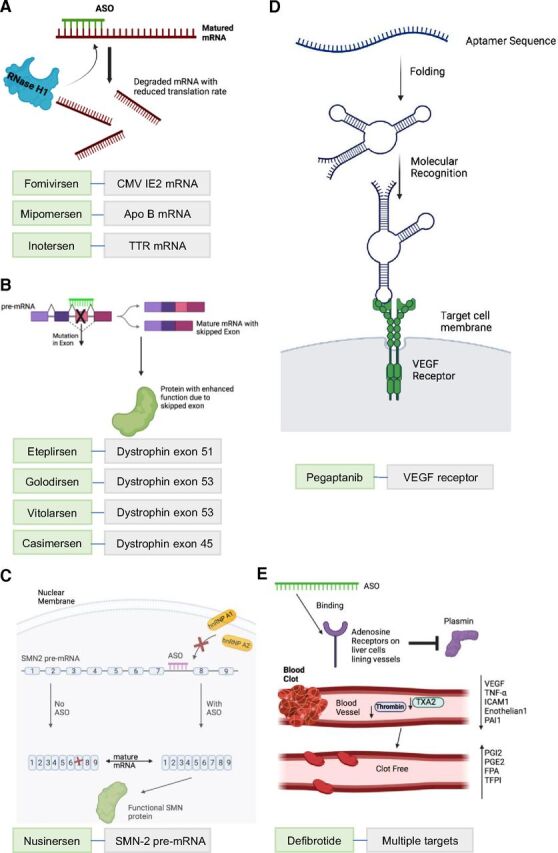
The mode of drug action of the 10 FDA-approved ASO drugs. (A) ASOs with RNase H-mediated mRNA degradation. By binding to their target mRNAs, ASO drugs cause the targeted mRNAs to be broken down into smaller pieces within the cell. ASO drugs in this modality are fomivirsen targeting on CMV IE2 mRNA, mipomersen targeting on Apo B mRNA, and inotersen targeting on transthyretin (TTR) mRNA. (B) ASO-mediated exon skipping. By sterically blocking a splicing site in the exon with mutations, ASO drugs result in skipping of the mutated exon and increased production of protein with enhanced function due to the skipped exon. ASO drugs in this mode include eteplirsen for exon 51 skipping, golodirsen and viltolarsen for exon 53 skipping, and casimersen for exon 45 skipping in Dystrophin mRNA. (C) ASO-mediated exon inclusion. By sterically blocking the splicing site in intron 7 of the SMN2 pre-mRNA, nusinersen blocks key intrinsic splicing factors heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) and heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2) and results in translation of full-length, functional SMN2 protein. (D) Aptamer ASO. Pegaptanib is a polynucleotide aptamer, which undergoes folding to bind to targeted vascular endothelial growth factor (VEGF). This prevents a cascade of kinase-mediated cell signaling, which then results in angiogenesis. (E) ASO with multiple modes. Defibrotide aims to bind to adenosine receptors and trigger a cascade of events. Plasmin is inhibited and can no longer hydrolyze clots. Key proclotting factors such as VEGF, tumor necrosis factor (TNF-α), intracellular adhesion molecule 1 (ICAM1), endothelium, plasminogen activator inhibitor 1 (PAI1), thromboxane A2 (TXA2), and thrombin are decreased. Anticlotting factors such as prostaglandins I2 and E2 and tissue factor pathway inhibitor (TFPI) all are increased. Fibrinopeptide A levels are increased due to chronic coagulation from disease. Clot-free blood vessels lead to general healing of damaged endothelial cells and tissue (Pescador et al., 2013).
