Abstract
Objectives
Increasingly, laboratories flag low serum alkaline phosphatase (sALP) that are age-and sex-specific in paediatrics. The aim of this study was to report clinical manifestations of paediatric patients with age-and sex-specific low sALP, thereby increasing awareness of its potential aetiologies.
Methods
This retrospective Canadian tertiary care paediatric hospital study assessed all sALP of ambulatory patients aged less than 18 years from 2015 to 2017. The hospital used a Beckman Coulter AU assay to measure sALP and compared values to the Canadian age-and sex-specific reference intervals from CALIPER. All children who had at least one subnormal age-and sex-specific sALP were evaluated. A review of medical charts of included patients was performed and demographic characteristics, medical history and diagnosis were collected, and categorized under groups of medical disorders.
Results
Of 11,874 included patients, 1,001 patients (9.2%) had low sALP. Of those, 48% (485/1,001) had transient low sALP activity and 9.6% (96/1,001) had persistently low sALP. Prolonged immobilization and inflammatory bowel disease represented the main aetiologies for persistently low sALP. Interestingly, 13.5% (13/96) of patients with persistently low sALP had no apparent aetiology.
Conclusions
Our results report aetiologies of low sALP in a Canadian paediatric population using age-and sex-specific Canadian reference ranges. This study highlights that healthcare providers should be aware that a low sALP may have clinical significance and should be repeated if warranted based on further clinical assessment.
Keywords: Alkaline phosphatase, Children, Laboratories, Paediatrics
Alkaline phosphatases (ALP) are a group of isoenzymes pro-duced by different organ systems in the body (1–3). There are four different types of isoenzymes found in humans; three of these four isoenzymes are tissue-specific to the intestine, placenta, and germ cells. The fourth ALP isoenzyme is present throughout the body, and thus is referred to as tissue non-specific alkaline phosphatase (TNSALP); however, it is particularly abundant in the liver, bone, and kidney (2,4). Commercially available laboratory tests for alkaline phosphatase measure all forms of ALP (5). In healthy adults, the bone and liver isoforms of TNSALP represent about 95% of measured ALP activity (6). Infants and children may have higher levels of the bone isoform of TNSALP, particularly during growth spurts (7). Levels differ significantly based on age and sex, and it is therefore important to establish and reference age- and sex-associated normal ranges (8).
Serum ALP (sALP) activity is often measured in clinical practice as part of routine studies. Physicians are typically alerted to elevated sALP activity by biochemistry laboratories, since high sALP is not an uncommon finding, and usually indicates hepatobiliary or bone disease (5,9,10). sALP activity can also be transiently elevated following a fracture or orthopaedic surgery (11,12). Increasingly, physicians are also being made aware of low sALP activity that are age- and sex-specific (13,14); however, they may not be aware of its clinical significance. It has been previously reported that low sALP can be indicative of a variety of conditions, including hypophosphatasia (HPP). HPP is a rare metabolic bone disease caused by deficient activity of TNSALP, resulting in the accumulation of inorganic pyrophosphate, which is a potent inhibitor of mineralization (1). Other reported causes of low sALP include malignancies, endocrine or metabolic disorders, inflammatory and renal diseases, nutritional deficiencies, and certain drugs. Furthermore, sALP activity can also be transiently low due to the variable course of these diseases, as well as therapeutics such as glucocorticoids, bisphosphonates and blood cell transfusions (5,15).
Given the myriad of medical conditions associated with low sALP, the primary aim of this retrospective study was to report clinical manifestations of ambulatory paediatric patients with age- and sex-specific low sALP activity, thereby increasing awareness of its aetiologies. The secondary objective was to determine whether a low sALP value normalized over time, or if it remained decreased which could be associated with a known aetiology or could be more concerning for an underlying condition such as HPP.
METHODS
Study design and population
This retrospective hospital-based data screening study was carried out at the Montreal Children’s Hospital, a paediatric tertiary hospital affiliated with McGill University. Ethical approval for this study was waived by the Research Ethics Board of the institution as it was based on chart reviews and considered as a quality improvement project.
All recorded sALP measurements of ambulatory patients aged less than 18 years between June 1st 2015 and December 31st 2017 were assessed, with the start date coinciding with the use of age- and sex-matched reference ranges at the newly constructed hospital site. The hospital used a Beckman Coulter AU biochemical assay to measure sALP and appropriate paediatric sALP reference ranges. The low sALP cut-off value was selected based on the lower limit of the age- and sex-specific reference range using a 90% confidence interval from the CALIPER study (see Supplementary Table) (16). As reference ranges for sALP activity are age-and sex-dependent, patients were grouped into seven different age categories. Since sALP values differ for sex at 13 years of age and above, these children were categorized in three groups based on sex. All children who had at least one sALP value detection below the lower reference limit for age and sex, defined as the 2.5th percentile lower reference limit, were evaluated for inclusion into this study. Inpatients were excluded due to increased risk of having low sALP values due to illness. Included children were then divided into three groups: (i) patients with only one available sALP value that was low (Group 1); (ii) transient low sALP –two or more sALP values that were low at least 6 months apart with prior or subsequent normal sALP values (Group 2); and (iii) persistently low sALP, defined as two or more sALP values that were low at least 6 months apart at different hospital visits without normalization (Group 3).
Measures
Following identification of the included children, a review of medical charts was performed. Children’s demographic characteristics, medical history and diagnosis were collected when available, and based on their manifestations, categorized according to known aetiologies of low sALP or with no associated medical history. In addition, any intervention(s) the patient required was documented including but not limited to medical treatment and/or surgical procedures and/or medically assistive devices.
Data analysis
Descriptive analysis was performed for all variables. Characteristics of children with low sALP values and their medical diagnosis were expressed as frequency and percentages. Data on sALP values were presented as median and range. Differences in sALP median values between children with persistently low sALP activity and medical condition were tested using the non-parametric Mann–Whitney U test. Statistical significance was based on probability values ≤0.05. All statistical analyses were conducted using Statistical Package for Social Sciences (SPSS, version 23.0, Chicago, IL, USA).
RESULTS
Over the 2.5-year study period, a total of 27,360 serum samples from 11,874 patients were analyzed for sALP activity. Based on the study exclusion criteria, 21,495 samples (10,834 patients) had a low cut-off ALP before applying the CALIPER references. A total of 1,001 patients (9.2%) had low sALP as per CALIPER reference intervals and were studied further. Of those, 42% (420/1,001) had only one low sALP measurement and almost half (48.5%; 482/1,001) had transient low sALP activity. Only 9.6% (96/1,001) of the patients had persistently low sALP activity (Figure 1).
Figure 1.
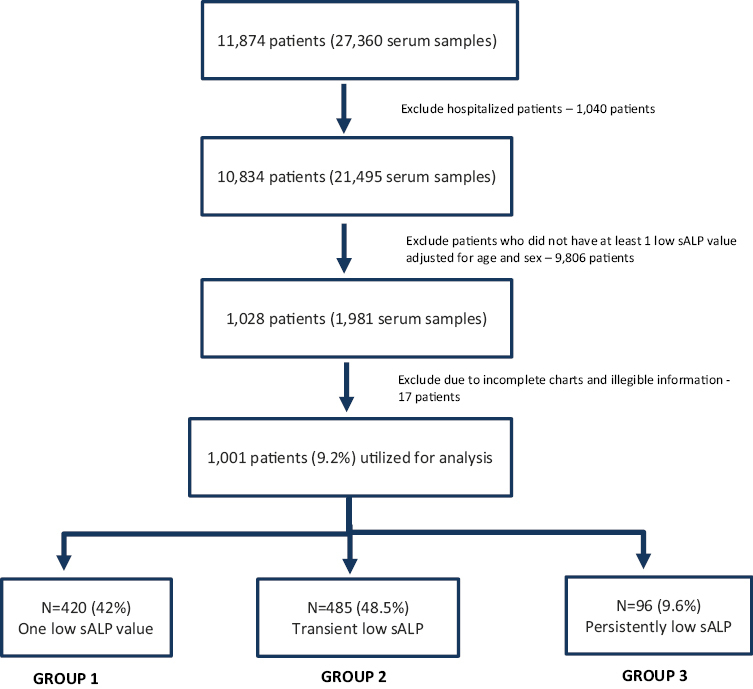
Flow chart of patients analyzed in the study
The age- and sex-specific sALP levels of included children are presented in Table 1. At the time of the investigation, sALP values ranged from 6 to 136 U/L. Fifty-six percent of the children were male and the average age was 9 years (SD 5.46).
Table 1.
Characteristics of patients with low alkaline phosphatase concentrations by age and sex (N = 1,001)
| Age range | 15 days to < 1 year | 1 to < 10 years | 10 to <13 years | 13 to < 15 years | 15 to <17 years | 17 to <19 years | |||
|---|---|---|---|---|---|---|---|---|---|
| Gender | * | * | * | M | F | M | F | M | F |
| Number of patients [N (%)] | 12 (1.2) | 535 (53.5) | 125 (12.5) | 49 (4.8) | 38 (3.8) | 78 (7.8) | 45 (4.5) | 62 (6.2) | 57 (5.7) |
| Lowest ALP value (U/L) [median (range)] | 91 (40–132) | 106 (5–132) | 95 (16–131) | 84 (32–119) | 65 (8–115) | 57 (20–106) | 39 (22–81) | 54 (5–74) | 36 (17–45) |
*No sex difference in the range
The aetiologies varied among the study participants (Table 2). Approximately 60% (607/1,001) of the patients with low sALP had an associated aetiology. However, most of the children with a single low sALP value (group 1) had no apparent diagnosis (75.9%; 319/420), whereas for group 2 (transiently low) and group 3 (persistently low), the majority of children had a well-known medical association [87.3% (423/485); 86.7%, (83/96), respectively]. Of the 27 patients with rheumatological conditions with low sALP values, 16 of them had Kawasaki disease (KD) (Table 2).
Table 2.
Patients with low sALP by group and medical condition (N = 1,001)
| Medical condition | Group 1† | Group 2‡ | Group 3§ |
|---|---|---|---|
| N = 420 | N = 485 | N = 96 | |
| Malnutrition | 27 (6.4) | 62 (12.7) | 11 (11.2) |
| Cancer | 6 (1.4) | 78 (16) | 13 (13.3) |
| Inflammatory bowel disease (IBD) | 0 | 105 (21.6) | 22 (22.4) |
| Prolonged immobilization | 5 (1.2) | 25 (5.1) | 26 (26.5) |
| Renal conditions | 6 (1.4) | 36 (7.4) | 3 (3.1) |
| Rheumatology conditions | 15 (3.5) | 9 (1.8) | 3 (3.1) |
| Skeletal issues | 1 (0.2) | 6 (1.2) | 2 (2) |
| Other | 41 (9.7) | 102 (21) | 3 (3.1) |
| No PMHx | 319 (75.9) | 62 (12.7) | 13 (13.3) |
All variables are presented as number (N) and percentage (%).
†Group 1: Patients with a single low sALP.
‡Group 2: Patients with at least 2 low sALP at least 6 months apart, with prior or subsequent normalization.
§Group 3: Patients with persistently low sALP at least 6 months apart without normalization
We identified 96 children with persistently low sALP activity (Table 3). The sALP did not vary by medical condition (P = 0.23; Table 3).Thirteen children (13.5%; 13/96) with persistently low sALP activity had no apparent diagnosis. No acknowledgement of the low sALP activity was documented in any of the patients’ charts.
Table 3.
Characteristics of patients with persistently low alkaline phosphatase concentrations by age and sex (N = 96)
| Medical condition [N (%)] | 15 days to < 1 year* | 1 to < 10 years* | 10 to <13 years* | 13 to < 15 years | 15 to <17 years | 17 to <19 years | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 53) | (N = 17) | M (N = 5) | F | M (N = 6) | F (N = 2) | M (N = 5) | F (N = 8) | |||
| Malnutrition | 11 (11.5) | 0 | 109 (59–128) | 0 | 0 | 0 | 0 | 36 (35–37) | 0 | 30 (23–37) |
| Cancer | 13 (13.5) | 0 | 89 (5–131) | 87 (51–113) | 0 | 0 | 0 | 0 | 0 | 0 |
| Inflammatory bowel disease (IDB) | 22 (22.9) | 0 | 95 (34–127) | 86 (77–92) | 92 (81–100) | 0 | 57 (47–69) | 0 | 38 (26–44) | 36 (36–37) |
| Prolonged immobilization | 26 (27.1) | 0 | 80 (37–129) | 59 (45–83) | 32τ | 0 | 36 (20–69) | 0 | 0 | 0 |
| Renal conditions | 3 (3.1) | 0 | 123τ | 79τ | 0 | 0 | 0 | 0 | 5 | 0 |
| Rheumatology conditions | 3 (3.1) | 0 | 91τ | 61τ | 0 | 0 | 0 | 0 | 0 | 30τ |
| Skeletal issues | 2 (2.1) | 0 | 97 (91–103) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 3 (3.1) | 0 | 88τ | 119τ | 0 | 0 | 0 | 0 | 0 | 40τ |
| No PMHx | 13 (13.5) | 0 | 88 (59–128) | 76 (47–103) | 74τ | 0 | 0 | 0 | 38 | 31 (26–37) |
ALP values (U/L) [median (range)].
M, Male; F, Female.
*No sex difference in the range.
τSingle patient in the category
DISCUSSION
Our study reports known aetiologies of low sALP activity in a Canadian paediatric population, and that a low sALP should be acknowledged as it may have some clinical significance. Novel to this study is the use of validated Canadian paediatric age- and sex-specific reference ranges to define low sALP activity in conjunction with available clinical data. Using these age- and sex-specific reference ranges, we found that 9.2% of children had low sALP activity, and that the majority had an associated aetiology. Of note, 16 patients had KD, which corroborates with a recently published case report of low sALP activity in this condition (17).
The number of patients in our study with low sALP activity is higher than what has been previously reported in the paediatric literature (13,18). Specifically, Bayramli et al. (18) reported low sALP activity in 0.9% of their included Turkish patient population over a study period of 17 months with an estimated 0.06% for persistently low sALP. This study used in house constructed age- and sex-specific sALP ranges with a 90% confidence interval. Although both studies were conducted at tertiary care paediatric hospitals where more chronically ill patients are likely to be assessed, the use of a different reference range for sALP measurement may have accounted for the discrepancy between our studies. It is also possible that sALP activity may not have been typically measured in conjunction with other liver enzyme tests as at our institution. Therefore, our patients with severe malnutrition, cancer, and IBD may have had sALP obtained or monitored more often, which can be associated with low sALP activity (19). Conversely, Deeb et al. (13) found a similar prevalence of low sALP (7.8% of their 2,890 patients) among children in the United Emerits. However, they used a North American age- and gender-specific low sALP reference range.
Our higher prevalence rate of low sALP may be explained by the use of the CALIPER reference ranges, a validated Canadian Pediatric Reference Database. The CALIPER reference intervals cover 95% of the healthy population, and therefore the lower limit encompasses otherwise healthy children. In our study, we were more likely to have sampled sicker patients on various medications with even lower sALP levels compared to the CALIPER lower cut-offs. Furthermore, these CALIPER reference ranges were obtained from samples of a multiethnic population of the greater Toronto area, including European and Asian descent but our measurements were procured from a Quebecois population. Therefore, we speculate that our paediatric cohort may have a unique genomic map post founder effect (20) and warrants further study. Lastly, EDTA contamination is known to decrease sALP activity (21). Although we cannot completely rule out this possibility in our study, our laboratory discards any known EDTA contaminated samples.
Prolonged immobilization and IBD represented the main aetiologies for children with persistently low sALP activity in this tertiary care paediatric cohort. Many of the immobilized patients were patients with Duchenne muscular dystrophy, treated with bisphosphonates and systemic glucocorticoids, which are two described causes of low sALP (13). None of our patients with persistently low sALP activity were already known to have HPP, a now treatable condition with varying severity associated with recurrent fractures, and premature tooth loss with the root intact. The carrier frequency of HPP in North America is approximately 1 in 200 (22) but is as high as 1 in 25 in Canadian Mennonites (23). As low sALP can be one of the first presenting signs of the less severe forms, health care providers should further assess abnormally low sALP values in the absence of an associated aetiology for signs suggestive of HPP with a targeted medical history and physical examination. Studies have also proposed screening with pyridoxal-5-phosphate levels as part of the diagnostic pathway for HPP (24,25).
Previous studies (14,18,25) were able to further identify patients via genetic testing with possible HPP based on initial screening by low sALP values, even prior to any onset of symptoms (26). Saraff et al. (14). reported 2 patients with ALPL mutations out of 50 patients with persistently low sALP activity, and Bayramli et al. (18). diagnosed 5 patients with HPP in a subset of 122 patients. Interestingly, 13.5% of our patients with persistently low sALP activity had no apparent aetiology. Given the size of our paediatric study, it is possible that our sample also included undetected cases of the less severe forms of HPP. However, considering the rarity of this condition, it is important to also consider other causes of persistently low sALP in the clinical context and to perform further step-wise investigations as previously suggested (14). As shown in our study, patients with transient low ALP values likely had fluctuations due to the variable course of their disease, with flares and remission, and the use of therapeutic agents, such as glucocorticoids, and transfusions/anaemia (5,13). Therefore, this supports the importance of performing a comprehensive clinical assessment and review of all previous laboratory values, avoiding the need for additional unnecessary investigations.
Of note, some patients in our study with low sALP activity had no apparent past medical history available in the chart, and did not have available repeat sALP values. While repeat values could have been obtained at a different laboratory, this is consistent with the perception that low sALP activity is generally not a cause of concern for treating health care professionals, as has been previously reported (13,14,18). Therefore, low sALP values should always be flagged to healthcare professionals for further clinical assessment.
There were many limitations to this study. First, this is a retrospective study, therefore limited to information gathered from the past. Past medical history was likely to be incomplete as some information may not have been judged relevant to the clinical visit, such as height, pubertal assessment and/or history of malnutrition and/or fractures. Additionally, it is possible that repeat sALP values were measured at a different test centre as part of a follow-up despite no documentation in the medical chart. Finally, there is also a selection bias in our sample of tertiary care paediatric patients as they are most likely to have their sALP measured and monitored.
In conclusion, our results increase awareness of aetiologies of low sALP activity in a Canadian pediatric population, and that a low sALP activity may have some clinical significance. A persistently low sALP may warrant a further targeted clinical and laboratory assessment, especially in the absence of an associated medical condition. Novel to this study is the use of validated Canadian paediatric age-and sex-specific reference ranges to define low sALP activity.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to express our gratitude to Dr. Fabienne Parente for her contributions to accessing biochemistry data. We also would like to thank Denis Thibeault for his contribution to biochemistry data extrapolation into Excel files.
Contributor Information
Anne Marie Sbrocchi, Department of Pediatrics, Montreal Children’s Hospital, Montreal; Faculty of Medicine, McGill University, Montreal.
Rosalie Cavin, Department of Pediatrics, Montreal Children’s Hospital, Montreal.
Annie Marleau, Division of Dentistry, Department of Pediatric Surgery, Montreal Children’s Hospital, Montreal; Faculty of Dental Medicine and Oral Health Sciences, McGill University, Montreal.
Tanya Fournier, Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, USA.
Michael Beecroft, Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, USA.
Beatriz Ferraz dos Santos, Division of Dentistry, Department of Pediatric Surgery, Montreal Children’s Hospital, Montreal; Faculty of Dental Medicine and Oral Health Sciences, McGill University, Montreal.
FUNDING
Funding for the conduct of this study was provided by Alexion Pharmaceuticals, Inc. The views expressed in this publication are those of the author(s) and not necessarily those of Alexion Pharmaceuticals, Inc. The funder had no role in the design and conduct of the study; collection, management and analysis of the data. The research team of the Montreal Children’s Hospital had full autonomy in all aspects of the study.
POTENTIAL CONFLICTS OF INTEREST
MB and TF report that they are employee of AZ—Alexion Rare Disease. The authors have no other disclosures.
REFERENCES
- 1.Millán JL, Whyte MP.. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int 2016;98(4):398–416. doi: 10.1007/s00223-015-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whyte MP. Hypophosphatasia: An overview for 2017. Bone 2017;102:15–25. doi: 10.1016/j.bone.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Mornet E. Hypophosphatasia. Best Pract Res Clin Rheumatol 2008;22(1):113–27. doi: 10.1016/j.berh.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Rockman-Greenberg C. Hypophosphatasia. Pediatr Endocrinol Rev 2013;10(Suppl 2):380–8. [PubMed] [Google Scholar]
- 5.McKiernan FE, Shrestha LK, Berg RL, Fuehrer J.. Acute hypophosphatasemia. Osteoporos Int 2014;25(2):519–23. doi: 10.1007/s00198-013-2447-x. [DOI] [PubMed] [Google Scholar]
- 6.Buchet R, Millán JL, Magne D.. Multisystemic functions of alkaline phosphatases. Methods Mol Biol 2013;1053:27–51. doi: 10.1007/978-1-62703-562-0_3. [DOI] [PubMed] [Google Scholar]
- 7.Whyte MP. Hypophosphatasia. In: Glorieus FH, Jeupnner H, Pettifor JM, eds. Pediatric Bone: Biology & Diseases. 3rd ed. San Diego: Elsevier (Academic Press); 2012;771–94.
- 8.Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: A CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012;58(5):854–68. doi: 10.1373/clinchem.2011.177741. [DOI] [PubMed] [Google Scholar]
- 9.Clinic M. ALP—clinical: Alkaline phosphatase serum. Available at: http://www.mayomedicallaboratories.com/test-catalog/clinical.
- 10.US National Library of Medicine. ALP—blood test. Available at: http://www.nlm.nih.gov/medlineplus. [DOI] [PubMed]
- 11.Whyte MP. Hypophosphatasia: Nature’s window on alkaline phosphatase function in man. In: Bilezikian JP, Raisz LG, Rodan, GA, eds. Principles of Bone Biology, 2ed, Academic Press,New York; 2002:1569–1599. doi:10.1016/B978-012098652-1.50172-4. [Google Scholar]
- 12.Hosking DJ. Changes in serum alkaline phosphatase after femoral fractures. J Bone Jt Surg Br 1978;60(1):61–5. doi: 10.1302/0301-620X.60B1.627581. [DOI] [PubMed] [Google Scholar]
- 13.Deeb A, Elfatih A.. Could alerting physicians for low alkaline phosphatase levels be helpful in early diagnosis of hypophosphatasia? J Clin Res Pediatr Endocrinol 2018;10(1):19–24. doi: 10.4274/jcrpe.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraff V, Narayanan VK, Lawson AJ, Shaw NJ, Preece MA, Högler W.. A diagnostic algorithm for children with low alkaline phosphatase activities: Lessons learned from laboratory screening for hypophosphatasia. J Pediatr 2016;172:181–186.e1. doi: 10.1016/j.jpeds.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Lum G. Significance of low serum alkaline phosphatase activity in a predominantly adult male population. Clin Chem 1995;41(4):515–8. [PubMed] [Google Scholar]
- 16.Abou El Hassan M, Stoianov A, Araújo PA, et al. CLSI-based transference of CALIPER pediatric reference intervals to Beckman Coulter AU biochemical assays. Clin Biochem 2015;48(16-17):1151–9. doi: 10.1016/j.clinbiochem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Padilla LA, Collins JL, Idigo AJ, Lau Y, Portman MA, Shrestha S.. Kawasaki disease and clinical outcome disparities among black children. J Pediatr 2021;229:54–60.e2. doi: 10.1016/j.jpeds.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayramli R, Cevlik T, Guran T, et al. Clinical significance of hypophosphatasemia in children. Calcif Tissue Int 2020;106(6):608–15. doi: 10.1007/s00223-020-00677-4. [DOI] [PubMed] [Google Scholar]
- 19.Semler O, Partsch CJ, Das AM, Prechtl A, Grasemann C.. Cross-sectional analysis: clinical presentation of children with persistently low ALP levels. J Pediatr Endocrinol Metab 2021;34(12):1559–66. doi: 10.1515/jpem-2021-0330. [DOI] [PubMed] [Google Scholar]
- 20.Scriver CR. Human genetics: Lessons from Quebec populations. Annu Rev Genom Hum Genet 2001;2:69–101. doi: 10.1146/annurev.genom.2.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Kalaria T, Ford C, Gama R.. Managing ethylenediaminetetraacetic acid (EDTA) interference in EDTA contaminated samples—selectivity in reporting analytes. Ann Clin Biochem 2023;60(2):92–9. doi: 10.1177/00045632221140989. [DOI] [PubMed] [Google Scholar]
- 22.Bissonnette B. Syndromes: Rapid Recognition and Perioperative Implications. 2e; McGraw Hill Professional; 2019. [Google Scholar]
- 23.Triggs-Raine B, Dyck T, Boycott KM, et al. Development of a diagnostic DNA chip to screen for 30 autosomal recessive disorders in the Hutterite population. Mol Genet Genom Med. 2016;4(3):312–21. doi: 10.1002/mgg3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Held CM, Guebelin A, Krebs A, et al. Screening for hypophosphatasia: Does biochemistry lead the way? J Pediatr Endocrinol Metab 2022;35(2):169–78. doi: 10.1515/jpem-2021-0104. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt T, Schmidt C, Amling M, Kramer J, Barvencik F.. Prevalence of low alkaline phosphatase activity in laboratory assessment: Is hypophosphatasia an underdiagnosed disease? Orphanet J Rare Dis 2021;16(1):452. doi: 10.1186/s13023-021-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araci MB, Akgun B, Atik T, et al. Clinical and molecular findings in children and young adults with persistent low alkaline phosphatase concentrations. Ann Clin Biochem 2021;58(4):335–41. doi: 10.1177/00045632211000102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


