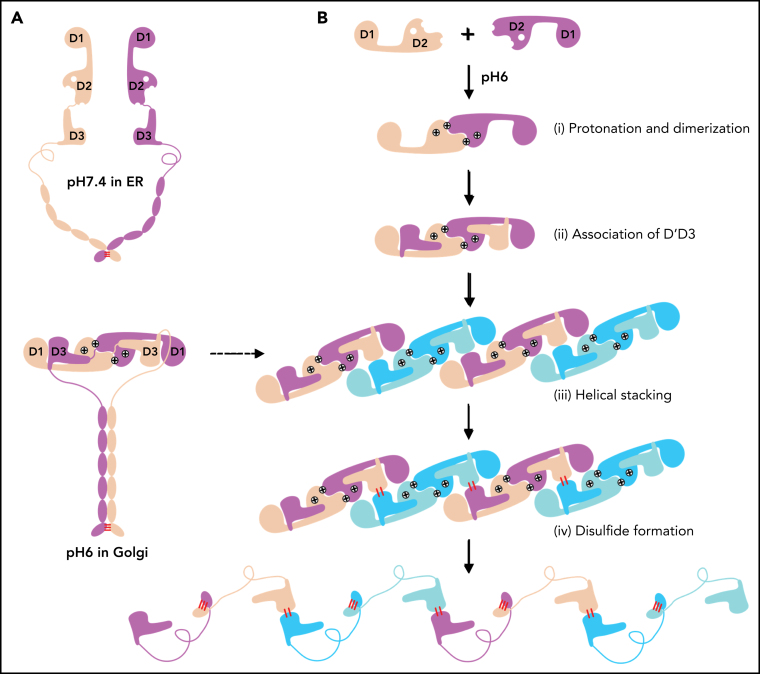
Figure 4.
The template mechanism of D1D2 in VWF multimerization and tubular storage. (A) Upon pH changes from ER to Golgi, the 2 histidine residues (His395 and His460) in D2 become protonated, and the N-terminal fragments of a pro-VWF dimer linked by the C-terminal CK domains would engage through the D2:D2 interface, forming a closed pro-VWF dimer in Golgi. (B) Two D1D2 molecules would form a pH-induced homodimer at pH6 (i) and then recruit 2 D'D3 monomers (ii). These D1D2 dimers loaded with 2 D'D3 molecules could then engage through the D1:D2 interface (iii) and align 2 D'D3 face-to-face, facilitating their intermolecular disulfide bond formation and helical packing of the repeating units (iv). The VWF multimers are then subject to tubular storage or release. The released VWF forms an extended head-to-head and tail-to-tail linear structure. All the D1D2 dimers loaded with D'D3 (ii-iv) could be transported into storage granules.