Figure 1: Recurrence of breast cancer (first invasive local, distant, or new contralateral primary) in the 15 trials with patient-level data comparing taxane plus anthracycline versus taxane without anthracycline.
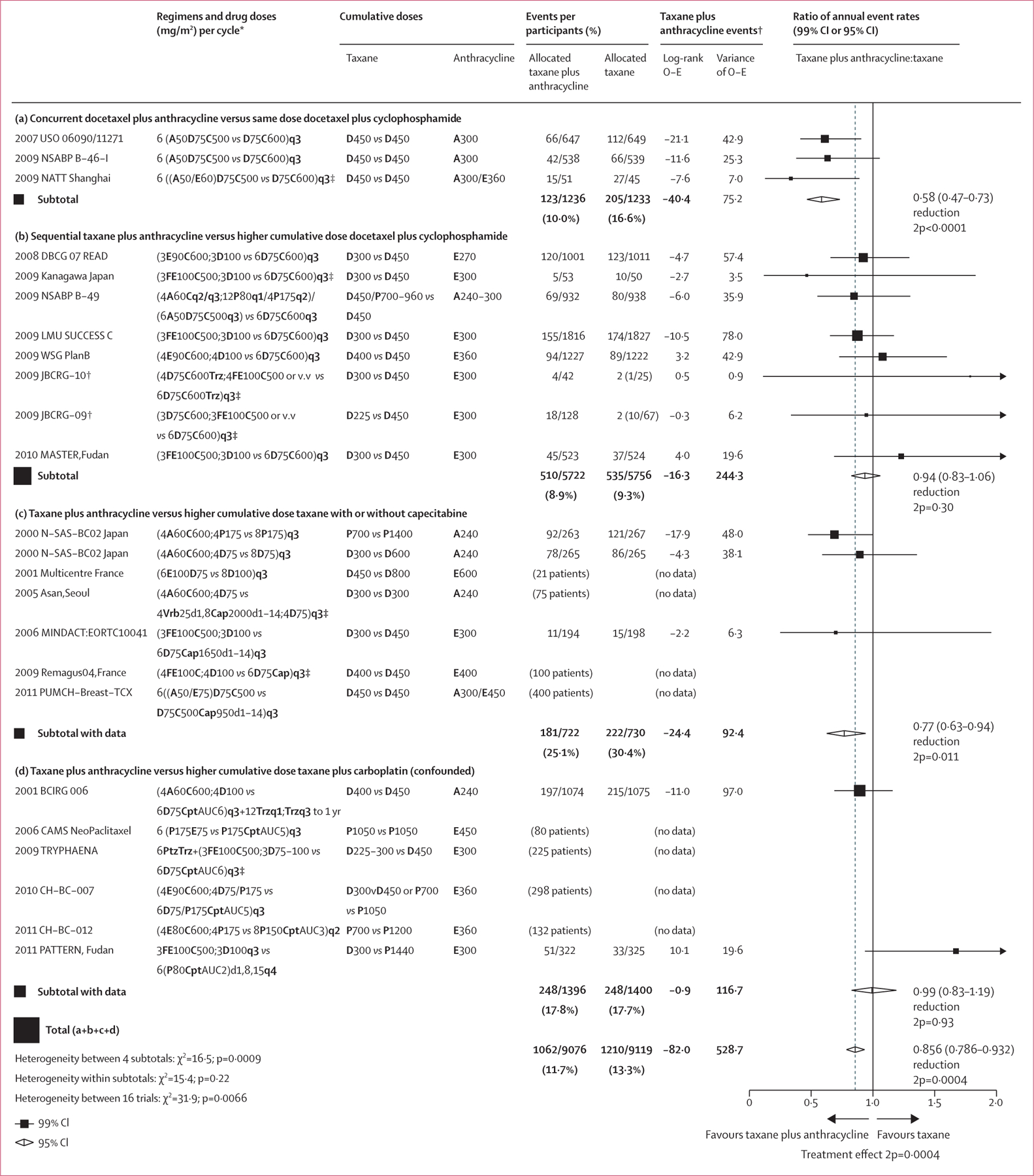
24 trials in total. One trial (N-SAS-BC 02) is shown on two lines as it was a 2 X 2 trial. Eight trials did not provide data. Taxanes were D and P. Anthracyclines were A and E. Other agents were C, F, M, Trz, Vrb, Cap, Cpt, and Ptz. 99% CIs are provided for individual trial data; 95% CIs are provided for subtotal and total data. A=doxorubicin. AUC=area under the curve. C=cyclophosphamide. Cap=capecitabine. Cpt=carboplatin. D=docetaxel. d=day of cycle. E=epirubicin. F=fluorouracil. M=methotrexate. O–E=observed minus expected. P=paclitaxel. Ptz=pertuzumab. q1=weekly. q2=every 2 weeks. q3=every 3 weeks. q4=every 4 weeks. Trz=trastuzumab. Vrb=vinorelbine. v.v=vice versa. yr=year. 2p=two-sided p value. *Any unstated doses are the same as for the non-anthracycline comparator. The regimens being compared in each study are described by the number of cycles, the drug abbreviation and dose in mg/m², and the frequency of the doses; a solidus (/) indicates or; a semicolon indicates then (sequential treatment). †For balance, control patients in three-way trials or trial strata count half or twice in subtotal(s) and in the final total of events and patients. ‡Pre-operative chemotherapy.
