Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease that is associated with anxiety and depression. Few studies have addressed interventions for symptoms of anxiety and depression in this population. To determine the efficacy of interventions for anxiety and depression in patients with AD. PubMed, MEDLINE, EMBASE, and PsycINFO were searched from inception to November 2023. English-language studies published in peer-reviewed journals evaluating the effect of interventions on anxiety and/or depression using validated assessment tools on patients with AD were included. Titles, abstracts, and articles were screened by at least two independent reviewers. Of 1410 references that resulted in the initial search, 17 studies were included. Fourteen of these studies are randomized controlled trials, while the other 3 studies are prospective controlled trials with pre and post-test designs. Data were extracted using a standardized extraction form, and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were followed. To accommodate trials with multiple interventions (each compared to a control group), we conducted a mixed-effects meta-analysis with the trial as a random effect. Prespecified outcomes were changes in symptoms of anxiety and depression in patients with AD as evaluated using standardized assessment tools. Of the 17 studies included in this systematic review, 7 pharmacological intervention studies with 4723 participants examining 5 different medications were included in a meta-analysis. Of these studies, only 1 study evaluated medications prescribed to treat anxiety and/or depression; the rest evaluated medications prescribed to treat AD. Meta-analysis of all the pharmacological interventions resulted in significant improvement in anxiety, depression, and combined anxiety-depression scale scores (standardized mean difference [95% CI]: − 0.29 [− 0.49 to − 0.09], − 0.27 [− 0.45 to − 0.08], − 0.27 [− 0.45 to − 0.08]) respectively. The 10 non-pharmacological studies with 2058 participants showed general improvement in anxiety but not depression. A meta-analysis of the non-pharmacological interventions was not conducted due to variable approaches and limited data. Pharmacological interventions designed to improve AD were found to improve anxiety and depression in patients with moderate-severe disease. More comprehensive studies on non-pharmacological and pharmacological interventions that primarily target anxiety and depression are needed.
Keywords: Atopic dermatitis, Eczema, Anxiety, Depression
Subject terms: Quality of life, Skin diseases
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease associated with profound physical and mental health symptoms1–4. There is a significantly higher prevalence of clinical depression (10.1% vs. 4.3%), anxiety disorder (17.2% vs. 11.1%), and suicidal ideation (12.7% vs. 8.3%) among patients with common skin diseases such as AD compared to general population5. Over time, adult patients tend to have fluctuating levels of depression that can wax and wane with AD symptoms. However, certain depressive symptoms, such as thoughts of self-harm, feeling bad, difficulty concentrating, and slow movement are persistent over time despite improvement in AD severity6. Additionally, adults with AD have significant impairments in their quality of life. Many patients report avoidance of social gatherings and activities, and loss of productivity at work or missing work due to their AD7–9. In the pediatric population, patients with severe AD symptoms have increased internalizing behaviors and depressive symptoms10. Moreover, caregivers, particularly those caring for children with severe AD, commonly experience symptoms of anxiety and depression11–13.
Although the mental health burden in patients with AD and their caregivers was well studied, few studies addressed interventions for patients with AD experiencing symptoms of anxiety and depression. Previous studies suggested that controlling AD severity may improve depressive symptoms in AD. However, some patients may have more persistent depressive symptoms and may need additional therapies or interventions6. In this paper, we review pharmacological and non-pharmacological interventions for patients experiencing symptoms of anxiety or depression.
Methods
Literature search and study selection
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines14. We conducted a systematic literature review search of interventional studies published through November 2023 in PubMed, MEDLINE, EMBASE, and PsycINFO using MESH or EMTREE terms “atopic dermatitis” or “eczema” and “anxiety” or “depression” (see Supplementary Table 1 for search details). In addition, reference lists of identified reviews and book chapters were also reviewed for additional studies. Unpublished manuscripts, conference proceedings/abstracts, case reports, and dissertations were not included. Title and abstract screening were conducted by two independent reviewers, with discrepancies resolved by discussion and consensus. A subsequent full-text review was conducted by four independent reviewers, and the decision to include or exclude the study was made by discussion and consensus.
Inclusion and exclusion criteria
We included all studies that met the following criteria: (a) published in English in a peer-reviewed journal, (b) participants were diagnosed with AD, (c) anxiety and/or depression were evaluated using validated assessment tools, (d) complete data (baseline data and end-point data) was accessible for analysis, and (e) sample size of the study was greater than or equal to 10 participants for each treatment arms to ensure the studies reviewed were adequately powered. Studies that measured health-related quality of life (HRQOL) were evaluated and included if anxiety or depression was part of the HRQOL assessment tool utilized. Both adult and pediatric studies are included in this review, and the findings are organized as pharmacological vs. non-pharmacological interventions. The complete review protocol has not been registered in advance and is available upon request.
Data extraction and quality assessment
We used a standardized extraction form to extract data, including reference of study, publication year, study design, patient characteristics, number of participating subjects, intervention, and outcome measures. The primary outcome we evaluated was a change in the level of anxiety and/or depression from baseline post-intervention. As multiple results may be available for different time points within the same study arms in the study reports, we used the following rules to extract data from each study. When possible, we extracted outcome data for anxiety and depression under each treatment arm to allow us to evaluate various dosages and treatment frequencies for pharmacological interventions. Some studies reported combined data for anxiety and depression, and these outcomes were analyzed separately. If data were available at multiple time points within the trial, we only used the data at the final time point of each intervention for meta-analysis. The risk of bias was assessed by evaluating the blinding of participants and personnel, allocation concealment, selective reporting, and incomplete outcome data using RoB215 and ROBINS-I16. Studies excluded due to small number of participants were also summarized to minimize potential bias.
Statistical analysis
Where data permitted, we planned to characterize the pooled effects from randomized controlled trials (RCT) of pharmacological or non-pharmacological treatments through meta-analyses. We performed meta-analyses using R 4.2 (R Core Team, Vienna, Austria) and the metafor package17. To accommodate trials with multiple interventions (each compared to a control group), we conducted mixed-effects meta-analysis with trial as a random-effect. We also fitted three-level models with intervention nested within the trial and unstructured covariance matrices to represent trials that compared several interventions to a common control group, but these models produced statistically indistinguishable results. Accordingly, we report the results of the simpler model. We estimated the pooled standardized mean difference (SMD) in outcomes (anxiety, depression, or combined anxiety-depression scale scores), in order to combine scores from different instruments and report results on a common effect size measure. We assessed heterogeneity using Cochran’s Q test. We examined funnel plots for publication bias and conducted meta-regressions to examine the impact of drug across all trials and the impact of dosage in Dupilumab trials. Due to the paucity of data from studies with non-pharmacological treatments, we were unable to perform meta-analyses and performed a narrative summary instead.
Results
Search results
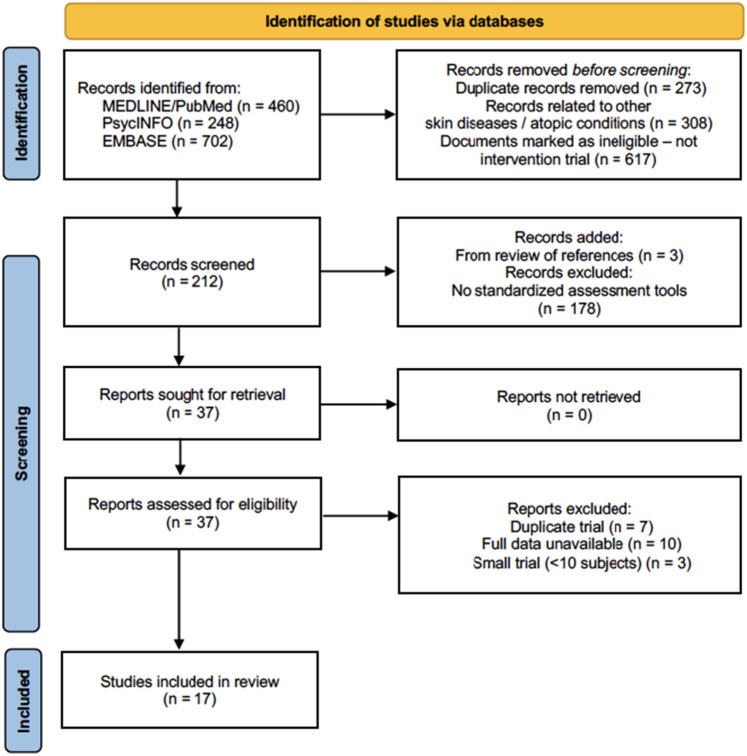
Our searches retrieved 1410 references, of which 212 were interventional studies (Fig. 1). Among these, we identified 37 studies evaluating the effects of pharmacological and non-pharmacological interventions on anxiety and/or depression in both adults and pediatric patients with AD. Two of the non-pharmacological interventions evaluated anxiety and depression in the caregiver18,19. Primary corresponding authors of these studies were contacted twice when data was not readily available from the manuscripts. Seven studies were excluded due to duplicated primary data20–26, while 10 studies were excluded due to data unavailability27–36. Three studies37–39 were excluded due to a small sample size (< 10 subjects), resulting in 17 final studies.
Figure 1.
PRISMA diagram of the study selection for this systematic review. Seventeen studies are included in this systematic review. Seven pharmaceutical interventions out of the seventeen studies are included in the meta-analysis.
Study characteristics
Pharmacological intervention
There are 7 pharmacological interventions identified by our systematic review (Table 1,40–46). Two of the pharmacological intervention studies were phase 2b RCT and five of the studies were phase 3 RCT. Studies conducted by Kawana, et al. were sponsored by a government grant while the rest of the RCTs were industry sponsored. Only one study evaluated the treatment of anxiety and/or depression using an anxiolytic/antidepressant (Tandospirone citrate (TC), which is a partial agonist of the 5-HT1A receptor and is more commonly used in China and Japan for the treatment of patients with anxiety disorders46. The six remaining studies are interventions to treat moderate-severe AD (Abrocitinib, Baricitinib, Dupilumab, Tralokinumab). All studies were conducted in adults; our literature search did not identify any pediatric pharmacological interventions.
Table 1.
Pharmacological intervention included in the meta-analysis.
| Author group (Ref) | Year | Study design | Drug evaluated | Study arms | Study length (weeks) | Study population | Sample Size (total) | Assess-ments used | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Thyssen et al.40 | 2022 | RCT | Abrocitinib and Dupilumab | Abrocitinib 100 mg q1d | 16 | Adults | 837 | HADS |
● Anxiety and depression significantly reduced in all treatment arms ● Abrocitinib 200 mg daily showed greatest reduction |
| Abrocitinib 200 mg q1d | |||||||||
| Dupilumab 300 mg q2w | |||||||||
| Placebo | |||||||||
| Thyssen et al.41 | 2022 | RCT | Baricitinib | Baracitinib 1 mg q1d | 16 | Adults | 1568 | HADS |
● Anxiety improved in all treatment arms compared to placebo ● Depression improved with Baricitinib 2 mg, 4 mg, and combination 4 mg + TCS group |
| Baracitinib 2 mg q1d | |||||||||
| Baracitinib 4 mg q1d | |||||||||
| Baracitinib 2 mg q1d + TCS | |||||||||
| Baracitinib 4 mg q1d + TCS | |||||||||
| Placebo | |||||||||
| Silverberg et al.42 | 2021 | RCT | Tralokinumab (TK) | TK 45 mg q2w | 12 | Adults | 204 | MCS | Improved the mental component summary at 12 weeks compared to placebo |
| TK 150 mg q2w | |||||||||
| TK 300 mg q2w | |||||||||
| Placebo | |||||||||
| DeBruin-Weller et al.43 | 2018 | RCT | Dupilumab | Dupilumab 300 mg q1w + TCS | 16 | Adults | 318 | HADS | HADS < 8 was achieved in the q2week group but was not achieved in the q1 week group |
| Dupilumab 300 mg q2w + TCS | |||||||||
| Placebo + TCS | |||||||||
| Simpson et al.44 | 2016 | RCT | Dupilumab | Dupilumab 300 mg q1w | 16 | Adults | 1379 | HADS | Anxiety and depression significantly reduced in all treatment arms |
| Dupilumab 300 mg q2w | |||||||||
| Placebo | |||||||||
| Simpson et al.45 | 2016 | RCT | Dupilumab | Dupilumab 300 mg q1w | 16 | Adults | 380 | HADS |
● Anxiety and depression significantly reduced at all doses and frequency ● 300 mg doses have the greatest efficacy in reducing anxiety and depression symptoms |
| Dupilumab 300 mg q2w | |||||||||
| Dupilumab 300 mg q4w | |||||||||
| Dupilumab 100 mg q4w | |||||||||
| Dupilumab 200 mg q4w | |||||||||
| Placebo | |||||||||
| Kawana et al.46 | 2010 | RCT | Tandospirone citrate (TC) | TC 30 mg q1d | 4 | Adults | 37 | POMS | Significant improvement in anxiety, depression, stress, and insomnia |
| Untreated |
RCT Randomized control trial, TCS topical corticosteroids, HADS Hospital Anxiety and Depression Scale (0–21 scale), POMS Profile of Mood States, MCS Mental component summary-summarized vitality, social functioning, role-emotional, and mental health domains.
The Profile of Mood States score (POMS)47, the Hospital Anxiety and Depression scale (HADS)48, and Short Form 36-Mental Component Summary (SF36v-MCS)49 were used to evaluate anxiety and depression outcomes. The POMS questionnaire assesses six mood subscales: tension-anxiety, depression, anger-hostility, vigor, fatigue, and confusion. Higher scores in these subscales excluding vigor indicate greater levels of anxiety and depression. HADS is a self-reported scale including 14 items (7 items each for anxiety and depression). A total subscale score of > 8 points out of possible 21 for each subscale indicates greater levels of anxiety and depression. SF36 is comprised of 36 questions and 9 domains that assess physical and mental health. The MCS domain assesses mental health domains, social function, vitality, and emotional health. The change in severity score for anxiety and depression is provided in Table 2.
Table 2.
The change in severity score for pharmaceutical intervention.
| Author group (Year) | Study length (weeks) | Sample size (group) | Study arms | Anxiety | Depression |
|---|---|---|---|---|---|
| Thyssen et al.40 | 16 | 238 | Abrocitinib 100 mg q1d | − 1.2 (− 1.6, -0.8) | -1.0 (-1.4, -0.7) |
| 226 | Abrocitinib 200 mg q1d | − 2.0 (− 2.4, -1.6) | -1.6 (-1.9, -1.2) | ||
| 241 | Dupilumab 300 mg q2w | − 1.5 (− 1.9, − 1.1) | − 1.2 (− 1.5, − 0.8) | ||
| 131 | Placebo | − 0.4 (− 0.9, 0.1) | − 0.3 (− 0.8, 0.2) | ||
| Thyssen et al.41 | 16 | 88 | Baracitinib 1 mg q1d | − 1.6 ± 0.8 | − 1.5 ± 0.9 |
| 78 | Baracitinib 2 mg q1d | − 1.9 ± 0.7 | − 2.7 ± 0.9 | ||
| 82 | Baracitinib 4 mg q1d | − 2.2 ± 0.7 | − 2.8 ± 0.8 | ||
| 60 | Baracitinib 2 mg q1d + TCS | − 1 ± 0.6 | − 2.1 ± 0.62 | ||
| 59 | Baracitinib 4 mg q1d + TCS | − 1.2 ± 0.52 | − 2.3 ± 0.66 | ||
| 162 | Placebo | − 0.9 ± 0.48 | − 0.3 ± 0.57 | ||
| 44 | Placebo + TCS | − 0.9 ± 0.58 | − 1.3 ± 0.70 | ||
| Silverberg et al.42 | 12 | 38 | TK 45 mg q2w | − 1.95 ± 0.31 | |
| 42 | TK 150 mg q2w | − 1.77 ± 0.30 | |||
| 42 | TK 300 mg q2w | 1.96 ± 0.30 | |||
| 38 | Placebo | − 0.71 ± 0.31 | |||
| DeBruin-Weller42 | 16 | 110 | Dupilumab 300 mg q1w + TCS | − 5.2 ± 5.56 | |
| 107 | Dupilumab 300 mg q2w + TCS | − 6.1 ± 5.59 | |||
| 108 | Placebo + TCS | − 2.3 ± 5.82 | |||
| Simpson et al.44 | 16 | 223 | Dupilumab 300 mg q1w* | − 5.2 ± 7.48 | |
| 224 | Dupilumab 300 mg q2w* | − 5.2 ± 7.47 | |||
| 224 | Placebo* | − 3 ± 4.19 | |||
| 239 | Dupilumab 300 mg q1w** | − 5.8 ± 4.33 | |||
| 233 | Dupilumab 300 mg q2w** | − 5.1 ± 4.27 | |||
| 236 | Placebo** | − 3 ± 4.3 | |||
| Simpson et al.45 | 16 | 63 | Dupilumab 300 mg q1w | − 2.2 ± 0.4 | − 2.4 ± 0.4 |
| 64 | Dupilumab 300 mg q2w | − 2.2 ± 0.4 | − 2.0 ± 0.4 | ||
| 65 | Dupilumab 300 mg q4w | − 1.3 ± 0.4 | 1.4 ± 0.4 | ||
| 65 | Dupilumab 100 mg q4w | − 1.4 ± 0.4 | − 1.0 ± 0.5 | ||
| 61 | Dupilumab 200 mg q4w | − 1.9 ± 0.4 | − 2.0 ± 0.5 | ||
| 61 | Placebo | − 0.4 ± 0.5 | 0.4 ± 0.5 | ||
| Kawana et al.46 | 4 | 20 | TC 30 mg q1d | − 2 | − 4.5 |
| 17 | Untreated | 0.8 | 0.2 | ||
Anxiety
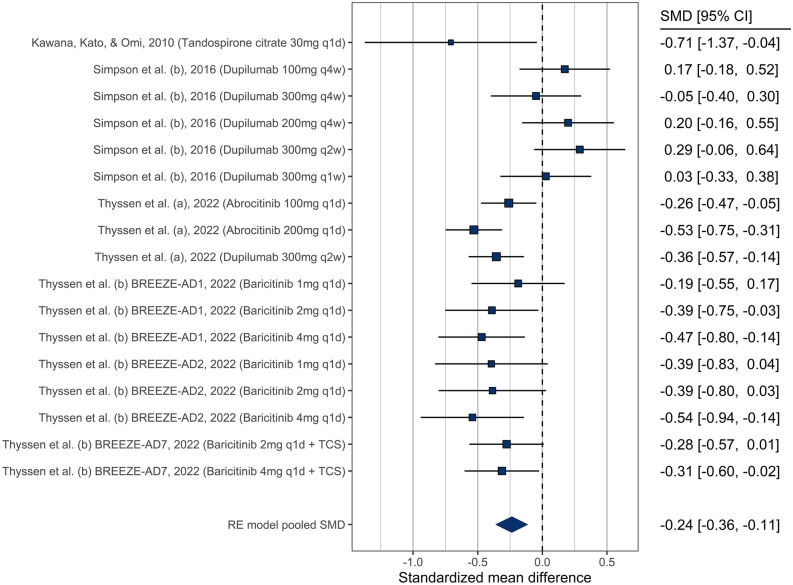
Of the studies related to anxiety, 7 evaluated multiple doses of the intervention or dosing frequencies resulting in 17 comparisons. Eight of these comparisons showed a statistically significant decrease in anxiety at the end of the study compared to baseline (Fig. 2). The meta-analysis indicated decreased anxiety levels in patients receiving pharmacological therapy for their AD: SMD = − 0.29, 95% CI [− 0.49 to − 0.09], with significant heterogeneity (Q = 38.8, p = 0.001). The funnel plot did not suggest publication bias (Supplementary Fig. 1), and neither type of drug nor dosage (only available for dupilumab) was a significant moderator of the effect (p = 0.34 and p = 0.67, respectively) (supplementary Fig. 2 and 3).
Figure 2.
Forest plot showing treatment effectiveness of various study arms in anxiety.
Depression
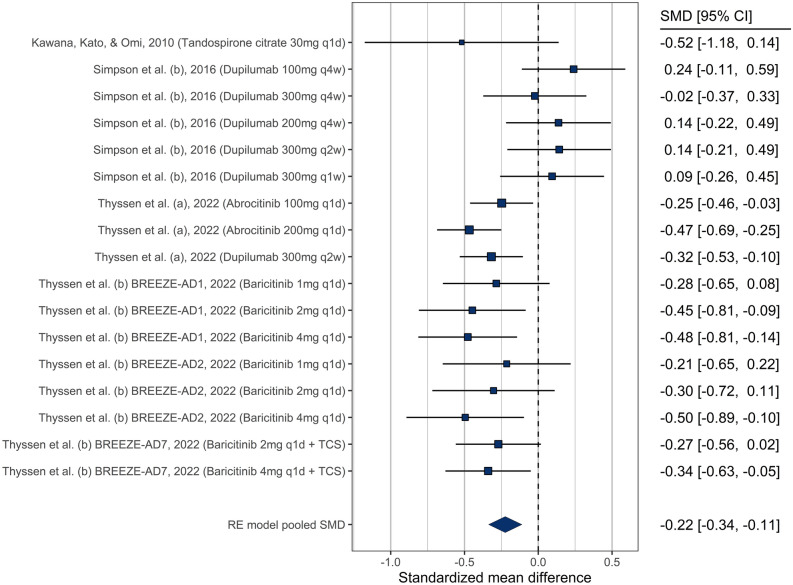
Similarly to anxiety, 7 studies related to depression evaluated multiple dosing regimens resulting in 17 comparisons. Seven comparisons showed a statistically significant decrease in depression by the end of the study compared to baseline (Fig. 3, see Table 1 for individual summary of each study). Overall, there was an improvement in depression severity in patients receiving pharmacological interventions for AD (SMD = − 0.27 [− 0.45 to − 0.08]), with significant heterogeneity (Q = 31.8, p = 0.01). We observed no publication bias (supplementary Fig. 1), and no significant moderation by drug (p = 0.36) or dosage (p = 0.75) (supplementary Fig. 2 and 3).
Figure 3.
Forest plot showing treatment effectiveness of various study arms in depression.
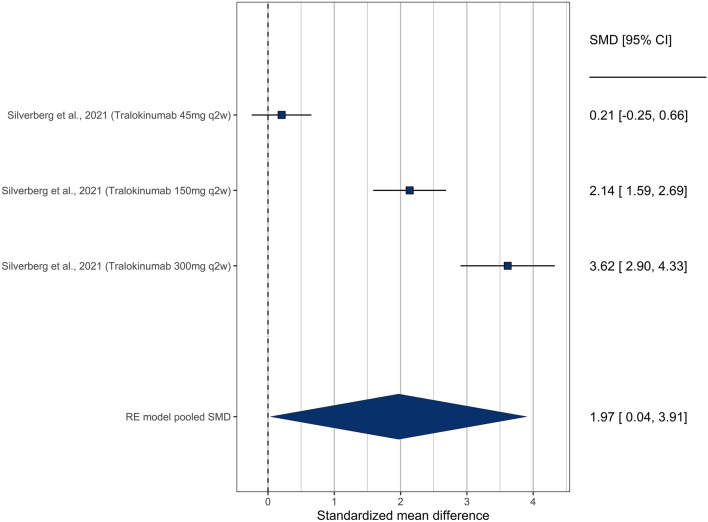
There was one study42 that did not specifically evaluate anxiety or depression, but rather assessed mental health domains using the SF36-MCS and should be considered as a surrogate measurement for depression. In this study, three unique dosages of Tralokinumab were evaluated. The 150 mg and 300 mg of tralokinumab showed significant improvement in MCS scores, while the 45 mg dosage did not. Overall, there was a significant improvement in MCS score (SMD = 1.49 [1.18–1.80]) (Fig. 4).
Figure 4.
Forest plot showing treatment effectiveness of various study arms in Silverberg et al.31,42. This study was graphed separately as it used SF-36v MCS to assess emotional and mental health, which we consider a surrogate measurement for depression.
Combined anxiety and depression
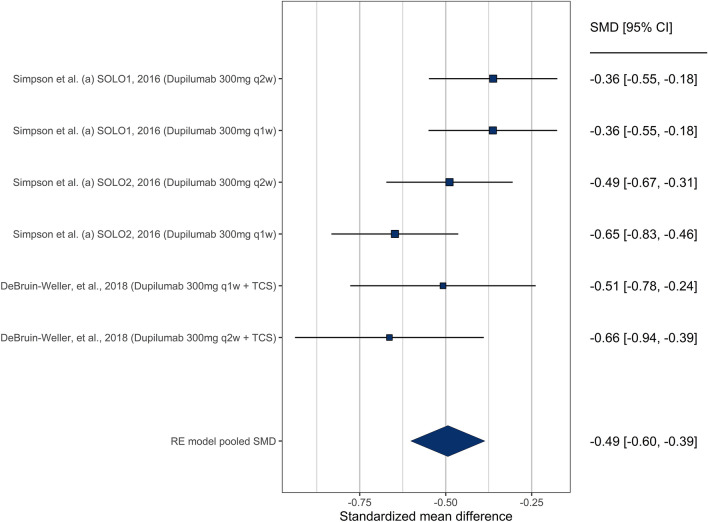
The combined HADS score was also evaluated in studies that did not provide sub-scores for anxiety and depression. There were 6 comparisons evaluating the change in HADS score from prior to initiation of the drug and at the end of the study. All 6 comparisons showed a statistically significant improvement in HADS score separately (Fig. 5), leading to an overall significant improvement in HADS score (SMD = − 0.50 [− 0.064 to − 0.35]). Heterogeneity was not significant (Q = 7.9, p = 0.16), and we observed no publication bias (supplementary Fig. 1). As all these studies involved dupilumab, we did not test for moderation by drug due to lack of moderation by dosage of dupilumab (p = 0.66).
Figure 5.
Forest plot showing treatment effectiveness of various study arms in both anxiety and depression combined.
Non-pharmacological intervention
We identified 10 non-pharmacological interventions (Table 3,18,19,50–57). Of these, there were 7 RCT, 1 non-randomized controlled trial, 1 open-label pilot clinical trial, and 1 prospective cohort study with pre- and post-test design with no control group. There were 6 adult studies18,19,51,52,56,57, 2 pediatric studies53,54, and 2 combined studies in both adults and children50,55. Sample sizes of these studies were generally small with significantly variable interventions.
Table 3.
Non-pharmacological intervention.
| Author group (Ref) | Year | Study design | Intervention evaluated | Study length (weeks) | Study population | Sample size (total) | Assess-ments used | Outcome |
|---|---|---|---|---|---|---|---|---|
| Kishimoto et al.57 | 2023 | RCT | Online mindfulness and self-compassion training | 13 | Adults | 107 | HADS | Significant improvement in anxiety (p < 0.001) and depression (p < 0.001) in the treatment group |
| Muzzolon et al.53 | 2021 | controlled, non-RCT | Educational intervention | 8–20 | Pediatric (14–18 yo) and caregivers | 48 | CDLQI & DFIQ | Significant improvement in the anxiety measures in both children and caregivers |
| Hedman-Lagerlof et al.51 | 2019 | Prospective, pre-and post-test design | Exposure based CBT | 10 | Adults | 9 | BAI & MADRS-S |
● Significant improvements in general anxiety (p < 0.005) ● No significant improvement in depression |
| Bae et al.50 | 2012 | RCT | Progressive muscle relaxation (PMR) | 4 | Adults and pediatrics | 25 | BDI & STAI | Significant improvement in depression and anxiety |
| Thomas et al.54 | 2011 | RCT | Ion-Exchange Water Softeners | 12 | Pediatrics | 336 | DFIQ | Significant improvement in depressive measures in DFIQ |
| Guerra-Tapia et al.55 | 2007 | RCT | Educational intervention (information leaflets) | 24 | Adults and pediatrics | 1247 | STAI |
● Significant improvement in anxiety compared to baseline ● No significant improvement in anxiety compared to patients receiving standard treatment |
| Linnet et al.52 | 2001 | RCT | Dynamic psychotherapy treatment | 24 | Adults | 32 | STAI | Significant improvement in anxiety only on patients with an initial high level of trait anxiety (TA) |
| Ehlers et al.56 | 1995 | RCT | Dermatologic education, relaxation therapy, CBT, combination education and CBT (DECBT), and standard medical care | 12 | Adults | 137 | STAI & CES-D |
● Significant reduction of anxiety after educational interventional program (both with and without behavioral therapy) ● No significant difference is observed for depression in all treatment group |
| Lin et al.19 | 2022 | RCT | Infant massages | 60 | Adults (mothers of infants with AD) | 97 | SAS & SDS | Significant improvement in SAS and SDS in mothers (p < 0.01) |
| Yoo et al.18 | 2018 | Prospective, pre-and post-test design | Educational session—in person and online | 8 | Adults (mothers of pediatric AD patients) | 20 | STAI | Significant improvement in anxiety at the end of the program (p < 0.001) |
CBT cognitive behavioral therapy, RCT Randomized control trial, BDI Beck Depression Inventory, STAI State-Trait Anxiety Inventory, BAI Beck Anxiety Inventory, MADRS-S Montgomery Åsberg Depression Rating Scale Self-report, CDLQI Children's Dermatology Life Quality Index, DFIQ the Dermatitis Family Impact Questionnaire, CES-D Center for Epidemiological Studies Depression Scale, SAS self-rating anxiety scale, SDS self-rating depression scale.
The non-pharmacological interventions consisted of educational interventions, psychotherapy, infant massages, progressive muscle relaxation (PMR), and use of ion-exchange water softener for improving eczema severity. Anxiety and depression were measured using HADS, Beck Depression Inventory (BDI)58, State-Trait Anxiety Inventory (STAI)59, Montgomery-Asperg Depression Scale (MADRS)60, Dermatitis Family Impact Questionnaire (DFI)61, Center for Epidemiological Studies Depression Scale (CES-D)62, Self-rating Anxiety Scale (SAS)63 and Self-rating Depression Scale (SDS)64.
Anxiety
Four studies (40%) evaluated educational interventions. Educational interventions are delivered as instructional leaflets, face-to-face sessions, online learning, or a combination of both and range between 8 to 24 weeks in duration. Patients and caregivers who received the educational intervention showed significant improvement in anxiety, except for the study by Guerra-Tapia et al.55. Although patients in the interventional arms of this study showed significant improvement in their anxiety compared to baseline, this is not significantly different compared to the standard treatment group at any time point throughout the study. Of note, this was the only study where the educational material was given as an instructional leaflet instead of an in-person session.
Four studies (40%) evaluated psychotherapy interventions either online or through in-person sessions. Kishimoto et al. evaluated an integrated online group therapy program of self-compassion and mindfulness for 13 weeks57. Hedman-Lagerlof et al. evaluated CBT, where patients were taught to refrain from scratching upon exposure to stressful situations in addition to mindfulness training51. Linnet et al. evaluated psychological sessions with a trained psychologist for 6 months52. Ehlers et al. evaluated CBT aimed at reducing scratching frequency as well as increasing patients’ ability to cope with itching and stress56. Of these 4 studies, Ehlers et al. only observed significant improvement in anxiety when CBT was combined with education, while the rest reported significant improvement in anxiety with psychotherapy alone, especially in patients with initial high levels of trait anxiety52.
Three studies (30%) evaluated relaxation techniques. Bae et al.50 evaluated 4 weeks of PMR in both adults and children, Ehlers et al.56 evaluated relaxation training based on auto-suggestive sessions led by clinical psychologists, and Lin et al.19 evaluated the effectiveness of infant massages. Patients who received PMR showed significant improvement in anxiety, but although patients who received relaxation training showed improvement in anxiety over baseline, it was not significant compared to patients receiving standard topical treatment alone. Lin et al. showed decreased anxiety in mothers who were taught to give once daily infant massages with skin oil for about 10 min. Although it is difficult to objectively evaluate anxiety and depression in infants, it should be noted that infants who received the massage interventions showed significant improvement in their caregiver reported quality of life scores in this study.
One study evaluated the use of ion-exchange water softener, and this study did not result in significant improvement in anxiety. All three studies evaluating caregivers showed significant improvement in the anxiety measures with educational interventions18,53 and infant massages19.
Depression
Neither educational or psychotherapy interventions resulted in significant improvement in depressive symptoms, whereas infant massages, PMR, and the use of ion-exchange water softener resulted in improvement in depressive symptoms. Meta-analysis was not conducted due to limited data available from the studies.
Excluded studies
We excluded three studies37–39 based on their small sample size. Cheirif-Wolosky et al. performed a retrospective analysis of children with AD between 2001 to 2018 who required systemic therapy for 3 months or longer37. Of the 21 children evaluated, 13 patients (61.9%) presented with anxiety and depression at baseline. Twelve patients received oral methotrexate, 9 received systemic steroids, 8 received oral thalidomide, 4 received azathioprine, 2 received mycophenolate mofetil, and 1 patient received cyclosporine. The duration of treatments ranged between 5.5 to over 20 months. As this is a retrospective study evaluating skin improvement as the primary outcome, anxiety and depression are not consistently evaluated across all treatment group. Wittkowski and Richards evaluated the benefits of 8-weeks cognitive behavioral therapy (CBT) in two adults with AD38. Both patients showed improvements in anxiety but only one patient showed improvement in depression. Modell et al. evaluated 3 weeks of Buproprion 150 mg/day and 300 mg/day dosages in an open-label study on 10 adult patients with AD and 10 adult patients with psoriasis (no placebo control arm) and showed skin improvement in 6 out of 10 AD patients (p = 0.0003) and 8 out of 10 patients with psoriasis (p = 0.001)39. They did not list any quantitative measures of anxiety and depression, which is also a criterion for exclusion in our meta-analysis.
Quality of individual study and risk of bias
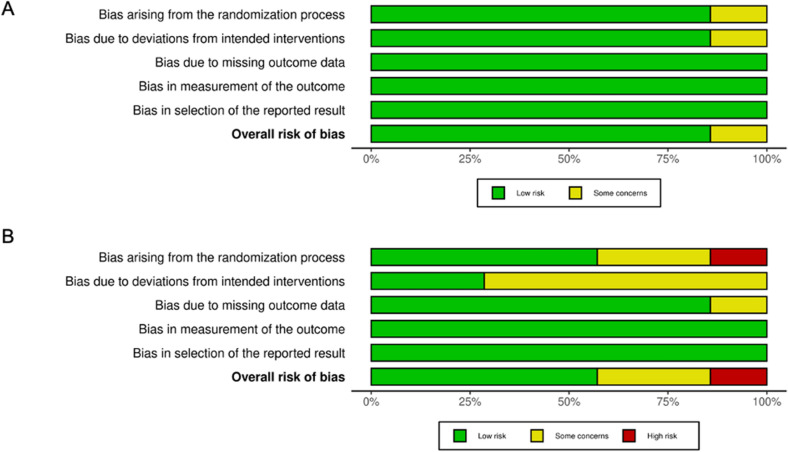
We assessed the risk of bias in individual studies using the Cochrane Risk of Bias tool15,16. There were generally fewer elements of high risk of bias in the newer studies compared to the older studies. As most of the pharmacological interventions evaluated were double blind RCT conducted after 2015, there is low concern for risk of bias in these studies (Fig. 6A). There are more elements with high risk of bias in the non-pharmacological interventions, associated with difficulty in blinding inherent in educational interventions and cognitive behavioral therapy treatment, as well as deviations from the intended protocol (Fig. 6B).
Figure 6.
Risk of bias of the pharmaceutical (A) and non-pharmaceutical interventions (B) based on 5 different domains.
Discussion
This is the first study to systematically review the impact of pharmacologic and non-pharmacologic interventions for AD on anxiety and depression symptoms in patients with AD. Patients with AD have increased levels of anxiety, depression, mental health hospitalizations, and increased suicidal ideations2,3,65–67. This mental health burden has been shown to be persistent over the course of the disease and is thought to wax and wane with the severity of AD symptoms6. Results from this systematic review indicate that although psychological interventions (both pharmacological and non-pharmacological) may play a key role in AD treatment, only few studies investigated the effects of such interventions in this patient population.
Pharmaceutical interventions
Various studies have shown that severe AD, pruritus, skin pain, and facial involvement have higher associations with anxiety and depression3,6,68. Given the persistence of mental health symptoms and the chronicity of AD, adequate control of AD symptoms may be effective in improving mental health comorbidities and the quality of life for patients with AD. We found that patients receiving pharmacological interventions aimed to control their AD symptoms showed a decrease in their levels of anxiety and depression in conjunction with the improvement of their skin. These results support the previous suggestions that improvement of AD severity may reduce mental health symptoms6.
While the first-line treatment for anxiety and depression includes selective serotonin/norepinephrine reuptake inhibitors (SSRI/SNRI)69, few studies have evaluated the efficacy of these medications in patients with AD. There was only one study46 in this review that systematically evaluated the use of an anxiolytic/antidepressant as a treatment for anxiety and depression in patients with AD. In addition to improvement in symptoms of anxiety and depression, there was also a significant decrease in the SCORAD index of the treated group compared to the untreated group. The investigator proposed inhibition of stress-induced mast cell degranulation as a possible mechanism for this effect based on their prior observation in the mouse model. Several case reports and small studies have observed more rapid and/or pronounced improvements in skin symptoms when anxiolytic/antidepressants were used in conjunction with common dermatologic therapy70–72. This is in support of how emotional stress can lead to AD exacerbation73,74. Larger studies are needed to evaluate the efficacy of antidepressant therapies in patients with AD not only as a treatment for anxiety and depression but also to determine their utility as an adjunct therapy for AD.
Most of the studies we included in this meta-analysis are clinical trials of drugs with relatively recent FDA approval intended for patients with moderate-severe eczema, with anxiety and depression as a secondary outcome. There was no RCT that evaluated the efficacy of topical medications or systemic glucocorticoids in improving anxiety and depression in patients with AD. This reflects the recent increased awareness of anxiety and depression as a co-morbidity associated with AD. However, as trials included in this study only evaluated patients with moderate-severe eczema, further research is needed to understand whether pharmacological interventions are equally effective for patients without moderate-severe disease or patients who do not qualify for immunomodulating therapies.
Non-pharmaceutical interventions
Compared to pharmacological interventions, there were fewer non-pharmacological interventional trials in the literature that addressed anxiety and depression in patients with AD. Interventions that incorporated in-person education and exposure-based CBT were effective in reducing anxiety in patients and caregivers as opposed to providing educational material alone, highlighting the necessity of interpersonal connection in the management of anxiety in patients with AD. Only one study56 did a head-to-head comparison between educational intervention, CBT, and relaxation techniques. Of these, only the educational program with or without CBT was shown to significantly improve anxiety.
Both education and exposure-based CBT did not show significant improvement in depression. Similar to our study, a systematic review of CBT and mindfulness techniques in patients with psoriasis showed improvement in anxiety levels but not in depression75. These findings suggest that depressive symptoms may be difficult to improve without improving the underlying disease. Of note, these studies focused on patients with moderate to severe disease. Therefore, conclusions on efficacy cannot be drawn for patients with mild or moderate disease. Furthermore, there are multiple meta-analyses illustrating the efficacy of CBT in improving both anxiety and depression in children, adolescents, and adult patients76–83. Thus, future studies in large, diverse populations are warranted to understand the efficacy of education and CBT in treating anxiety and depression in patients with AD.
Relaxation and stress reduction techniques may have a role in the treatment of AD-related anxiety and depression, but few studies have rigorously evaluated these strategies84,85. PMR is a muscle relaxation therapy that has been shown effective in treating anxiety disorders, including panic and generalized anxiety disorders86,87. Bae, et al. found that PMR intervention consisting of physical and mental components for 4 weeks significantly reduced anxiety and depression. Several other case series, both in adults and pediatrics, have shown similar results30,88.
Ion exchange water softeners were shown to be effective in improving depression in AD despite lack of improvement in primary outcome of the study as measured by eczema severity measured by Six Area Six Sign Atopic Dermatitis Score (SASSAD)51. Decreased depression symptoms despite the absence of objective improvement in eczema might be due to perceived improvement of eczema in the setting of significant change in patient-oriented eczema scores reported in the study. Interestingly, a similar effect was not observed for anxiety in the study. This may indicate that anxiety/depression can be multifactorial in nature including diet, lifestyle, gut microbiome, and genetic influences, rather than dependent on eczema severity alone89,90. Further work is needed to understand this difference and how this may play into mental health and quality of life in patients with eczema.
Our results support other systematic reviews and meta-analyses on mental health interventions for patients with other dermatological diseases. A previous study found that patients taking isotretinoin for acne had significant improvement in depressive symptoms after treatment91. Fleming et al.92 demonstrated that patients with moderate-severe psoriasis had significant improvement in depression after treatment with a biologic. We hypothesized that there might be a positive correlation between the improvement in AD markers and psychological metrics that could be tested if we had access to individualized data from each trial. Among several possibilities, this correlation could be linear with a set improvement in anxiety or depression stemming from some equivalent improvement in itch or rash, or psychological improvement may be felt only after a certain threshold of improvement in itch or rash was met. Currently, available data cannot conclude whether a patient suffering from severe AD would be expected to have complete resolution of the related psychological burdens should his or her AD symptoms significantly improve. Therefore, future studies should consider releasing participant-level results so this needed analysis can be performed.
It should be noted that none of the pharmacological studies included in this systematic review evaluated anxiety and depression as a primary outcome. Future studies evaluating topical vs systemic AD treatment in improving anxiety and depression, as well as the use of anti-anxiety and/or anti-depressants in patients with severe AD would be of tremendous value. Linnet et al. indicate that patients with baseline high anxiety levels seemed to be the ones who benefited the most from non-pharmaceutical interventions. Further research is needed to identify AD patients who will benefit the most from either pharmaceutical or non-pharmaceutical interventions. Further research is also needed to understand patients’ and caregivers’ preferences for pharmacologic and non-pharmacologic interventions for AD in addition to the relative efficacy of these different approaches. Access to mental health providers, particularly those who understand AD might be a barrier for patients who prefer non-pharmacologic interventions. Given that anxiety and depression associated with AD are contextual and related to the experience of living with AD symptoms, referral to mental health providers who lack knowledge of the condition may not be helpful93.
The present study has other limitations. As mentioned previously, the pharmacological interventions were conducted on patients with moderate/severe AD. Since anxiety and depression are usually evaluated as a secondary outcome, there are several studies that we excluded due to incomplete data despite attempts to request data from the study investigators. Furthermore, these pharmacological studies do not preclude or determine if enrolled patients are already receiving pharmacological treatment or therapy for their anxiety and/or depression. There was some heterogeneity in the techniques and/or tools used, as well as the design of studies included in the non-pharmacological treatment as they are evaluating very disparate interventions (i.e. use of water softener vs cognitive behavioral therapy). This precludes us from conducting similar meta-analyses for non-pharmacological interventions. Overall, this work offers the first systematic review of the pharmacologic and non-pharmacologic treatments for anxiety and depression in the AD patient population. Our findings indicate that patients with moderate to severe AD may directly benefit from treatments targeting the mental health burdens related to AD, even in cases where the AD symptoms are stable.
Conclusion
In conclusion, pharmacological interventions intended to improve AD severity in patients with moderate to severe disease are also effective in decreasing anxiety and depression. Despite the significant mental health burden of AD, there are a limited number of studies evaluating the efficacy of interventions targeting anxiety and depression in this population. This underscores the need for future RCT in large, diverse patient populations to determine whether different pharmacological and non-pharmacological interventions can improve anxiety and depression in patients with AD.
Supplementary Information
Author contributions
K.C. and I.A.M. conceived the presented idea. S.P.H., S.C., O.A., S.K., and A.S. designed the study, gathered all the data and performed the analysis. S.P.H. and S.C. wrote the main manuscript text and prepared the tables and figures 1 and 6. A.S. prepared figures 2, 3, 4 and 5. K.C., I.A.M. and J.I.S. aided in interpreting the results and provided critical feedback. All authors reviewed the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stella P. Hartono and Sheena Chatrath.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-59162-9.
References
- 1.Kage P, Simon JC, Treudler R. Atopic dermatitis and psychosocial comorbidities. J. Dtsch. Dermatol. Ges. 2020;18(2):93–102. doi: 10.1111/ddg.14029. [DOI] [PubMed] [Google Scholar]
- 2.Cheng BT, Silverberg JI. Depression and psychological distress in US adults with atopic dermatitis. Ann. Allergy Asthma Immunol. 2019;123(2):179–185. doi: 10.1016/j.anai.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br. J. Dermatol. 2019;181(3):554–565. doi: 10.1111/bjd.17683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu SH, Silverberg JI. Association between atopic dermatitis and depression in US adults. J. Invest. Dermatol. 2015;135(12):3183–3186. doi: 10.1038/jid.2015.337. [DOI] [PubMed] [Google Scholar]
- 5.Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, Jemec GBE, et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Invest. Dermatol. 2015;135(4):984–991. doi: 10.1038/jid.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatrath S, Lei D, Yousaf M, Chavda R, Gabriel S, Silverberg JI. Longitudinal course and predictors of depressive symptoms in atopic dermatitis. J. Am. Acad. Dermatol. 2022;87(3):582–591. doi: 10.1016/j.jaad.2022.04.061. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Chan TC, Lin YC, Cho YT, Tang CH, Chu CY. Impact of atopic dermatitis on work and activity impairment in Taiwan. Acta Derm. Venereol. 2021;101(9):adv00556. doi: 10.2340/00015555-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capozza K, Schwartz A, Lang JE, Chalmers J, Camilo J, Abuabara K, et al. Impact of childhood atopic dermatitis on life decisions for caregivers and families. J. Eur. Acad. Dermatol. Venereol. 2022;36(6):e451–e454. doi: 10.1111/jdv.17943. [DOI] [PubMed] [Google Scholar]
- 10.Kern C, Wan J, LeWinn KZ, Ramirez FD, Lee Y, McCulloch CE, et al. Association of atopic dermatitis and mental health outcomes across childhood: A Longitudinal Cohort Study. JAMA Dermatol. 2021;157(10):1200–1208. doi: 10.1001/jamadermatol.2021.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su W, Chen H, Gao Y, Qin Q, Liu B, Deng W, et al. Anxiety, depression and associated factors among caretakers of children with atopic dermatitis. Ann. Gen. Psychiatry. 2022;21(1):12. doi: 10.1186/s12991-022-00389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim RW, Barta K, Begolka WS, Capozza K, Eftekhari S, Tullos K, et al. The quantitative impact of atopic dermatitis on caregivers across multiple life domains. Br. J. Dermatol. 2022;187(6):1041–1043. doi: 10.1111/bjd.21855. [DOI] [PubMed] [Google Scholar]
- 13.Capozza K, Gadd H, Kelley K, Russell S, Shi V, Schwartz A. Insights from caregivers on the impact of pediatric atopic dermatitis on families: "I'm Tired, Overwhelmed, and Feel Like I'm Failing as a Mother". Dermatitis. 2020;31(3):223–227. doi: 10.1097/DER.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 18.Yoo JB, De Gagne JC, Jeong SS, Jeong CW. Effects of a hybrid education programme for Korean mothers of children with atopic dermatitis. Acta Derm. Venereol. 2018;98(3):329–334. doi: 10.2340/00015555-2862. [DOI] [PubMed] [Google Scholar]
- 19.Lin L, Yu L, Zhang S, Liu J, Xiong Y. The positive effect of mother-performed infant massage on infantile eczema and maternal mental state: A randomized controlled trial. Front. Public Health. 2022;10:1068043. doi: 10.3389/fpubh.2022.1068043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cork MJ, Eckert L, Simpson EL, Armstrong A, Barbarot S, Puig L, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: Analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J. Dermatol. Treat. 2020;31(6):606–614. doi: 10.1080/09546634.2019.1612836. [DOI] [PubMed] [Google Scholar]
- 21.Thaci D, Simpson EL, Deleuran M, Kataoka Y, Chen Z, Gadkari A, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: A pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2) J. Dermatol. Sci. 2019;94(2):266–275. doi: 10.1016/j.jdermsci.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Wollenberg A, Nakahara T, Maari C, Peris K, Lio P, Augustin M, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 Phase 3 randomized trial. J. Eur. Acad. Dermatol. Venereol. 2021;35(7):1543–1552. doi: 10.1111/jdv.17278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blauvelt A, Guttman-Yassky E, Paller AS, Simpson EL, Cork MJ, Weisman J, et al. Long-term efficacy and safety of Dupilumab in adolescents with moderate-to-severe atopic dermatitis: Results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE) Am. J. Clin. Dermatol. 2022;23(3):365–383. doi: 10.1007/s40257-022-00683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lio PA, Simpson EL, Han G, Soung J, Ball S, Sun L, et al. Improvement in sleep and itch and enhanced quality of life in adult patients with moderate-to-severe atopic dermatitis: Results from a phase 3 trial of baricitinib therapy. J. Dermatol. Treat. 2022;33(4):2057–2062. doi: 10.1080/09546634.2021.1914308. [DOI] [PubMed] [Google Scholar]
- 25.Yosipovitch G, de Bruin-Weller M, Armstrong A, Wu JJ, Herranz P, Thaçi D, et al. Dupilumab treatment provides sustained improvements over 2 years in symptoms and quality of life in adults with atopic dermatitis. Dermatol. Therapy. 2021;11(6):2147–2157. doi: 10.1007/s13555-021-00630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrelo A, Rewerska B, Galimberti M, Paller A, Yang CY, Prakash A, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in paediatric patients with moderate-to-severe atopic dermatitis with an inadequate response to topical corticosteroids: results from a phase III, randomized, double-blind, placebo-controlled study (BREEZE-AD PEDS) Br. J. Dermatol. 2023;189(1):23–32. doi: 10.1093/bjd/ljad096. [DOI] [PubMed] [Google Scholar]
- 27.Janmohamed SR, Oranje AP, Devillers AC, Rizopoulos D, van Praag MC, Van Gysel D, et al. The proactive wet-wrap method with diluted corticosteroids versus emollients in children with atopic dermatitis: A prospective, randomized, double-blind, placebo-controlled trial. J. Am. Acad. Dermatol. 2014;70(6):1076–1082. doi: 10.1016/j.jaad.2014.01.898. [DOI] [PubMed] [Google Scholar]
- 28.Miniotti M, Lazzarin G, Ortoncelli M, Mastorino L, Ribero S, Leombruni P. Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of Dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol. Ther. 2022;35(5):e15407. doi: 10.1111/dth.15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricci G, Bendandi B, Aiazzi R, Patrizi A, Masi M. Three years of Italian experience of an educational program for parents of young children affected by atopic dermatitis: Improving knowledge produces lower anxiety levels in parents of children with atopic dermatitis. Pediatr. Dermatol. 2009;26(1):1–5. doi: 10.1111/j.1525-1470.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- 30.Schachner L, Field T, Hernandez-Reif M, Duarte AM, Krasnegor J. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr. Dermatol. 1998;15(5):390–395. doi: 10.1111/j.1525-1470.1998.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 31.Silverberg JI, Thyssen JP, Simpson EL, Yosipovitch G, Stander S, Valdez H, et al. Impact of oral abrocitinib monotherapy on patient-reported symptoms and quality of life in adolescents and adults with moderate-to-severe atopic dermatitis: A pooled analysis of patient-reported outcomes. Am. J. Clin. Dermatol. 2021;22(4):541–554. doi: 10.1007/s40257-021-00604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashizume H. How bet to fight that nasty itch—From new insights into the neuroimmunological, neuroendocrine, and neuro-physiological bases of pruritus to novel therapeutic approaches. Exp. Dermatol. 2005;14:225–240. doi: 10.1111/j.0906-6705.2005.0321a.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu YJ, Wu WF, Hung CW, Ku MS, Liao PF, Sun HL, et al. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J. Microbiol. Immunol. Infect. 2017;50(5):684–692. doi: 10.1016/j.jmii.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Chan S, Cornelius V, Cro S, Harper JI, Lack G. Treatment effect of omalizumab on severe pediatric atopic dermatitis: The ADAPT randomized clinical trial. JAMA Pediatr. 2020;174(1):29–37. doi: 10.1001/jamapediatrics.2019.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whalley D, Huels J, McKenna SP, Van Assche D. The benefit of pimecrolimus (Elidel, SDZ ASM 981) on parents' quality of life in the treatment of pediatric atopic dermatitis. Pediatrics. 2002;110(6):1133–1136. doi: 10.1542/peds.110.6.1133. [DOI] [PubMed] [Google Scholar]
- 36.Miniotti M, Ribero S, Mastorino L, Ortoncelli M, Gelato F, Bailon M, et al. Long-term psychological outcome of patients with moderate-to-severe atopic dermatitis continuously treated with Dupilumab: Data up to 3 years. Exp. Dermatol. 2023;32(6):852–858. doi: 10.1111/exd.14786. [DOI] [PubMed] [Google Scholar]
- 37.Cheirif-Wolosky O, Elizalde-Jimenez IG, Romero MTG. Systemic treatment for severe atopic dermatitis in children: a case series. Bol. Med. Hosp. Infantil Mex. 2022;79(5):310–317. doi: 10.24875/BMHIM.22000002. [DOI] [PubMed] [Google Scholar]
- 38.Wittkowski A, Richards HL. How beneficial is cognitive behaviour therapy in the treatment of atopic dermatitis? A single-case study. Psychol. Health Med. 2007;12(4):445–449. doi: 10.1080/13548500601109268. [DOI] [PubMed] [Google Scholar]
- 39.Modell JG, Boyce S, Taylor E, Katholi C. Treatment of atopic dermatitis and psoriasis vulgaris with bupropion-SR: A pilot study. Psychosom. Med. 2002;64(5):835–840. doi: 10.1097/01.psy.0000021954.59258.9b. [DOI] [PubMed] [Google Scholar]
- 40.Thyssen JP, Yosipovitch G, Paul C, Kwatra SG, Chu CY, DiBonaventura M, et al. Patient-reported outcomes from the JADE COMPARE randomized phase 3 study of abrocitinib in adults with moderate-to-severe atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2022;36(3):434–443. doi: 10.1111/jdv.17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thyssen JP, Lio P, Ball S, Pierce E, Sun L, Chen Y, et al. Improvement in symptoms of anxiety and depression in patients with atopic dermatitis after treatment with baricitinib. J. Eur. Acad. Dermatol. Venereol. 2022;36(2):e147–e150. doi: 10.1111/jdv.17704. [DOI] [PubMed] [Google Scholar]
- 42.Silverberg JI, Guttman-Yassky E, Gooderham M, Worm M, Rippon S, O'Quinn S, et al. Health-related quality of life with tralokinumab in moderate-to-severe atopic dermatitis: A phase 2b randomized study. Ann. Allergy Asthma Immunol. 2021;126(5):576–83 e4. doi: 10.1016/j.anai.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 43.de Bruin-Weller M, Thaci D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE) Br. J. Dermatol. 2018;178(5):1083–1101. doi: 10.1111/bjd.16156. [DOI] [PubMed] [Google Scholar]
- 44.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 45.Simpson EL, Gadkari A, Worm M, Soong W, Blauvelt A, Eckert L, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): A phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD) J. Am. Acad. Dermatol. 2016;75(3):506–515. doi: 10.1016/j.jaad.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 46.Kawana S, Kato Y, Omi T. Efficacy of a 5-HT1a receptor agonist in atopic dermatitis. Clin. Exp. Dermatol. 2010;35(8):835–840. doi: 10.1111/j.1365-2230.2009.03771.x. [DOI] [PubMed] [Google Scholar]
- 47.Reddon JR, Marceau R, Holden RR. A confirmatory evaluation of the profile of mood states—Convergent and discriminant item validity. J. Psychopathol. Behav. 1985;7(3):243–259. doi: 10.1007/BF00960756. [DOI] [Google Scholar]
- 48.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Measurement properties of the hospital anxiety and depression scale used in atopic dermatitis in adults. J. Invest. Dermatol. 2019;139(6):1388–1391. doi: 10.1016/j.jid.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 49.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. 1992;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bae BG, Oh SH, Park CO, Noh S, Noh JY, Kim KR, et al. Progressive muscle relaxation therapy for atopic dermatitis: Objective assessment of efficacy. Acta Derm. Venereol. 2012;92(1):57–61. doi: 10.2340/00015555-1189. [DOI] [PubMed] [Google Scholar]
- 51.Hedman-Lagerlof E, Bergman A, Lindefors N, Bradley M. Exposure-based cognitive behavior therapy for atopic dermatitis: An open trial. Cogn. Behav. Ther. 2019;48(4):300–310. doi: 10.1080/16506073.2018.1504320. [DOI] [PubMed] [Google Scholar]
- 52.Linnet J, Jemec GB. Anxiety level and severity of skin condition predicts outcome of psychotherapy in atopic dermatitis patients. Int. J. Dermatol. 2001;40(10):632–636. doi: 10.1046/j.1365-4362.2001.01272.x. [DOI] [PubMed] [Google Scholar]
- 53.Muzzolon M, Imoto RR, Canato M, Abagge KT, de Carvalho VO. Educational intervention and atopic dermatitis: Impact on quality of life and treatment. Asia Pac. Allergy. 2021;11(2):e21. doi: 10.5415/apallergy.2021.11.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas KS, Koller K, Dean T, O'Leary CJ, Sach TH, Frost A, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: The Softened Water Eczema Trial (SWET) Health Technol. Assess. 2011;15(8):1–156. doi: 10.3310/hta15080. [DOI] [PubMed] [Google Scholar]
- 55.Guerra-Tapia A, Lleonart M, Balañá M. Observational study to evaluate the impact of an educational/informative intervention in the emotional status (anxiety) of patients with atopic dermatitis (CUIDA-DEL) Actas Dermo-Sifiliograficas. 2007;98(4):250–258. doi: 10.1016/S0001-7310(07)70058-3. [DOI] [PubMed] [Google Scholar]
- 56.Ehlers A, Strangier U, Gieler U. Treatment of atopic-dermatitis—A comparison of psychological and dermatological approaches to relapse prevention. J. Consult. Clin. Psychol. 1995;63(4):624–635. doi: 10.1037/0022-006X.63.4.624. [DOI] [PubMed] [Google Scholar]
- 57.Kishimoto S, Watanabe N, Yamamoto Y, Imai T, Aida R, Germer C, et al. Efficacy of integrated online mindfulness and self-compassion training for adults with atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2023;159(6):628–636. doi: 10.1001/jamadermatol.2023.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Personal. Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 59.Metzger RL. Reliability and validity study of state-trait anxiety inventory. J. Clin. Psychol. 1976;32(2):276–278. doi: 10.1002/1097-4679(197604)32:2<276::AID-JCLP2270320215>3.0.CO;2-G. [DOI] [Google Scholar]
- 60.Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Asberg Depression Scale: Reliability and validity. Acta Psychiatr. Scand. 1986;73(5):544–548. doi: 10.1111/j.1600-0447.1986.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 61.Dodington SR, Basra MK, Finlay AY, Salek MS. The Dermatitis Family Impact questionnaire: A review of its measurement properties and clinical application. Br. J. Dermatol. 2013;169(1):31–46. doi: 10.1111/bjd.12232. [DOI] [PubMed] [Google Scholar]
- 62.Hautzinger M. The CES-D scale: A depression-rating scale for research in the general population. Diagnostica. 1988;34(2):167–173. [Google Scholar]
- 63.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 64.Zung WW. A self-rating depression scale. Arch. Gen. Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 65.Hsu DY, Smith B, Silverberg JI. Atopic dermatitis and hospitalization for mental health disorders in the United States. Dermatitis. 2019;30(1):54–61. doi: 10.1097/DER.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sandhu JK, Wu KK, Bui TL, Armstrong AW. Association between atopic dermatitis and suicidality: A systematic review and meta-analysis. JAMA Dermatol. 2019;155(2):178–187. doi: 10.1001/jamadermatol.2018.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicholas MN, Gooderham MJ. Atopic dermatitis, depression, and suicidality. J. Cutan. Med. Surg. 2017;21(3):237–242. doi: 10.1177/1203475416685078. [DOI] [PubMed] [Google Scholar]
- 68.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic dermatitis in america study: A cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J. Invest. Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 69.Coplan JD, Aaronson CJ, Panthangi V, Kim Y. Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World J. Psychiatry. 2015;5(4):366–378. doi: 10.5498/wjp.v5.i4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danilin I, Ziewozinska Z, Korsunskaya I, Artemieva M, Suleymanov R, Sokolov V, et al. Low dose chlorprothixene administration in patients with atopic dermatitis. Eur. Psychiatry. 2019;56:S351. [Google Scholar]
- 71.Bajwa WK, Stevens CA. The resolution of eczema during treatment with lamotrigine: A case report. Primary Care Companion J. Clin. Psychiatry. 2012;14(3):27233. doi: 10.4088/PCC.11l01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown DG, Bettley FR. Psychiatric treatment of eczema: a controlled trial. Br. Med. J. 1971;2(5764):729–734. doi: 10.1136/bmj.2.5764.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashizume H, Horibe T, Ohshima A, Ito T, Yagi H, Takigawa M. Anxiety accelerates T-helper 2-tilted immune responses in patients with atopic dermatitis. Br. J. Dermatol. 2005;152(6):1161–1164. doi: 10.1111/j.1365-2133.2005.06449.x. [DOI] [PubMed] [Google Scholar]
- 74.Yang T, Huang X, Xu J, Situ M, Xiao Q, Kural KC, et al. Explore the underlying mechanism between atopic dermatitis and major depressive disorder. Front. Genet. 2021;12:640951. doi: 10.3389/fgene.2021.640951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zill JM, Christalle E, Tillenburg N, Mrowietz U, Augustin M, Harter M, et al. Effects of psychosocial interventions on patient-reported outcomes in patients with psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2019;181(5):939–945. doi: 10.1111/bjd.17272. [DOI] [PubMed] [Google Scholar]
- 76.Karyotaki E, Efthimiou O, Miguel C, Bermpohl FMG, Furukawa TA, Cuijpers P, et al. Internet-based cognitive behavioral therapy for depression: A systematic review and individual patient data network meta-analysis. JAMA Psychiatry. 2021;78(4):361–371. doi: 10.1001/jamapsychiatry.2020.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zakhour S, Nardi AE, Levitan M, Appolinario JC. Cognitive-behavioral therapy for treatment-resistant depression in adults and adolescents: A systematic review. Trends Psychiatry Psychother. 2020;42(1):92–101. doi: 10.1590/2237-6089-2019-0033. [DOI] [PubMed] [Google Scholar]
- 78.Li JM, Zhang Y, Su WJ, Liu LL, Gong H, Peng W, et al. Cognitive behavioral therapy for treatment-resistant depression: A systematic review and meta-analysis. Psychiatry Res. 2018;268:243–250. doi: 10.1016/j.psychres.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 79.Christ C, Schouten MJ, Blankers M, van Schaik DJ, Beekman AT, Wisman MA, et al. Internet and computer-based cognitive behavioral therapy for anxiety and depression in adolescents and young adults: Systematic review and meta-analysis. J Med Internet Res. 2020;22(9):e17831. doi: 10.2196/17831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, Zhou X, Zhou C, Zhang Y, Pu J, Liu L, et al. Efficacy and acceptability of cognitive behavioral therapy for depression in children: A systematic review and meta-analysis. Acad. Pediatr. 2017;17(1):9–16. doi: 10.1016/j.acap.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Wickersham A, Barack T, Cross L, Downs J. Computerized cognitive behavioral therapy for treatment of depression and anxiety in adolescents: Systematic review and meta-analysis. J Med Internet Res. 2022;24(4):e29842. doi: 10.2196/29842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hall J, Kellett S, Berrios R, Bains MK, Scott S. Efficacy of cognitive behavioral therapy for generalized anxiety disorder in older adults: Systematic review, meta-analysis, and meta-regression. Am. J. Geriatr. Psychiatry. 2016;24(11):1063–1073. doi: 10.1016/j.jagp.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatry. 2008;69(4):621–632. doi: 10.4088/JCP.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ersser SJ, Cowdell F, Latter S, Gardiner E, Flohr C, Thompson AR, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst. Rev. 2014;2014(1):CD004054. doi: 10.1002/14651858.CD004054.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oska C, Nakamura M. Alternative psychotherapeutic approaches to the treatment of eczema. Clin. Cosmet. Investig. Dermatol. 2022;15:2721–2735. doi: 10.2147/CCID.S393290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conrad A, Roth WT. Muscle relaxation therapy for anxiety disorders: it works but how? J. Anxiety Disord. 2007;21(3):243–264. doi: 10.1016/j.janxdis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Gaylord C, Orme-Johnson D, Travis F. The effects of the transcendental mediation technique and progressive muscle relaxation on EEG coherence, stress reactivity, and mental health in black adults. Int. J. Neurosci. 1989;46(1–2):77–86. doi: 10.3109/00207458908991618. [DOI] [PubMed] [Google Scholar]
- 88.Hosono S, Fujita K, Nimura A, Akita K. Release of cervical muscle tension improves psychological stress and symptoms of moderate-to-severe atopic dermatitis: A case series with 20 patients. Dermatol. Therapy. 2022;12(10):2383–2395. doi: 10.1007/s13555-022-00814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng QX, Lim YL, Yaow CYL, Ng WK, Thumboo J, Liew TM. Effect of probiotic supplementation on gut microbiota in patients with major depressive disorders: A systematic review. Nutrients. 2023;15(6):1351. doi: 10.3390/nu15061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davies MR, Kalsi G, Armour C, Jones IR, McIntosh AM, Smith DJ, et al. The Genetic Links to Anxiety and Depression (GLAD) Study: Online recruitment into the largest recontactable study of depression and anxiety. Behav. Res. Ther. 2019;123:103503. doi: 10.1016/j.brat.2019.103503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li C, Chen J, Wang W, Ai M, Zhang Q, Kuang L. Use of isotretinoin and risk of depression in patients with acne: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e021549. doi: 10.1136/bmjopen-2018-021549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fleming P, Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, et al. Effect of biologics on depressive symptoms in patients with psoriasis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2015;29(6):1063–1070. doi: 10.1111/jdv.12909. [DOI] [PubMed] [Google Scholar]
- 93.Chong AC, Schwartz A, Lang J, Ong PY, Myles IA, Silverberg JI, et al. Patients' and Caregivers' preferences for mental health care and support in atopic dermatitis. Dermatitis. 2023;35:S70–S76. doi: 10.1089/derm.2023.0111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.