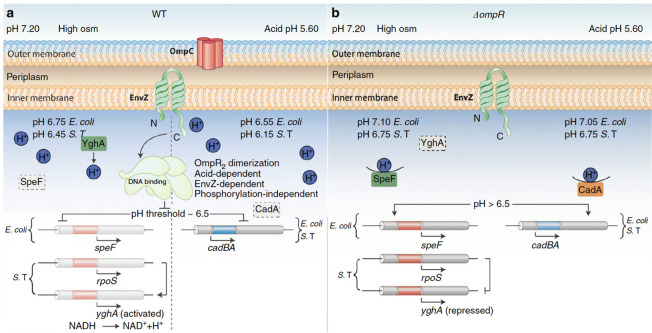
Fig 2.
A summary of the role of OmpR in the acid stress response and acidification of the cytoplasm in response to osmolytes. Under acid and osmotic stress conditions, both S. Typhimurium and E. coli are acidified in an OmpR-dependent manner. OmpR requires n interaction with EnvZ, but not phosphorylation, to bring about cytoplasmic acidification. (A) In wild-type bacteria, under acid stress (right), OmpR represses the cad operon to eliminate proton consumption, resulting in acidification. At high osmolality, in S. Typhimurium, OmpR represses rpoS to relieve RpoS repression of yghA, producing protons (left). In E. coli, OmpR represses ornithine decarboxylase (speF), enabling cytoplasmic acidification. The pH optima of CadA is 6.1–6.5 (15); this optima contributes to a threshold of response. At pH 6.5 and below (achieved during acid stress), OmpR represses the cad operon, resulting in acidification. At high osmolality (pH 6.75, E. coli; 6.45, S. Typhimurium), acidification is less because the CAD system is working to restore neutrality. Acidification occurs through proton production (S. Typhimurium) or repression of a different amino acid decarboxylation system (E. coli). Intracellular acidification is required for activating SPI-2-dependent effector secretion. EnvZ senses and responds to cytoplasmic acidification via helix–coil transitions. A physical interaction of OmpR with EnvZ drives a conformational change, resulting in unphosphorylated OmpR dimer formation. EnvZ-dependent OmpR dimerization creates an active OmpR2 interface, favoring DNA binding and subsequent repression. (B) In the ompR null strain, during acid stress, CadC/BA is expressed and drives amino acid decarboxylation. This process consumes intracellular protons, restoring cytoplasmic pH and maintaining intracellular pH homeostasis. At high osmolality, in an ompR null S. Typhimurium strain, RpoS represses yghA, preventing proton release, resulting in a neutralized cytoplasm. In an ompR null E. coli strain, activated SpeF eliminates cytoplasmic protons during ornithine decarboxylation, maintaining pH homeostasis. Reprinted from reference 2.