Fig. 4 |. Efficacy and safety of C3.MPS LNPs in Idua-W392X neonates.
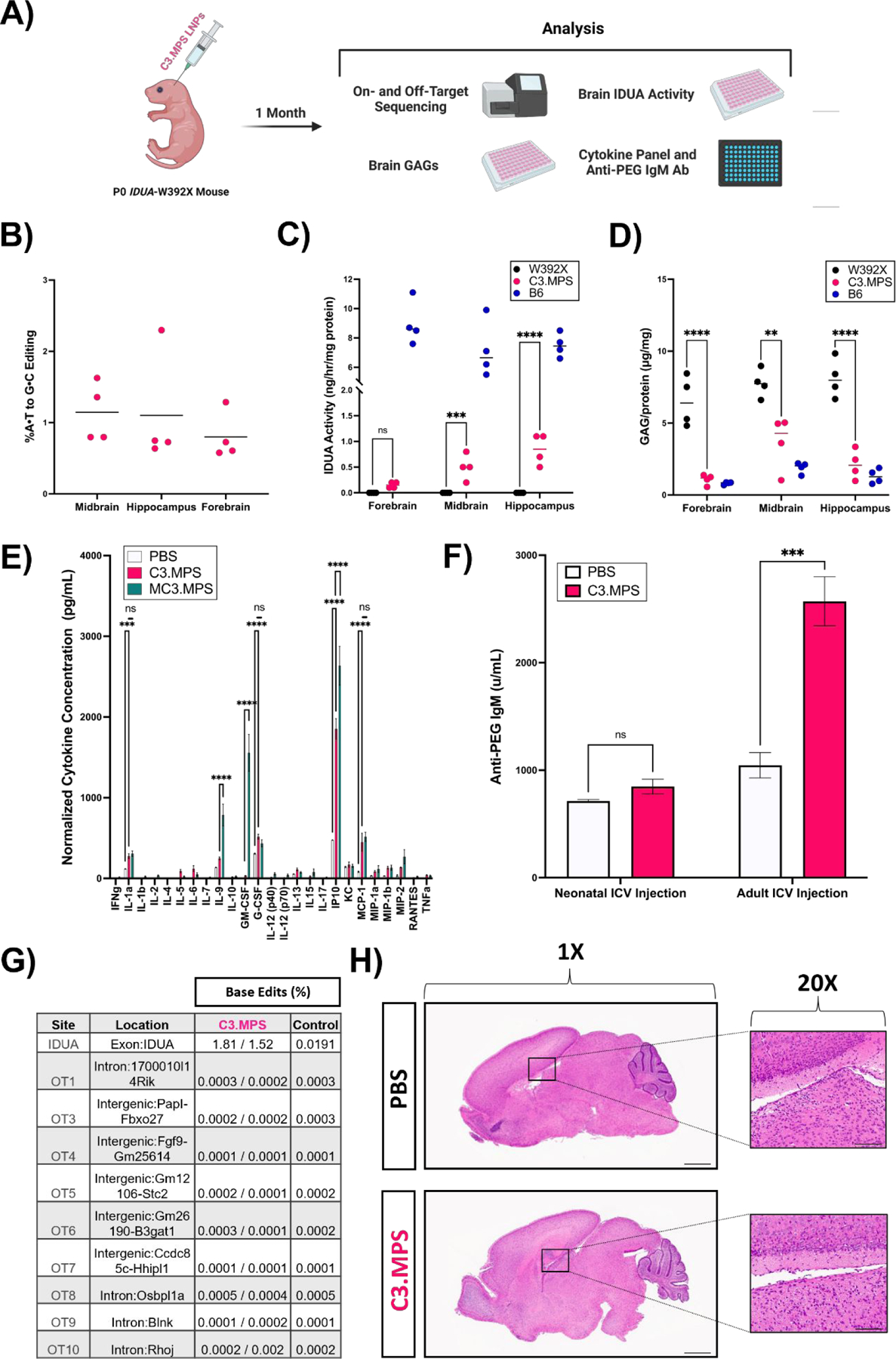
(A) Scheme for genetic, biochemical, and safety analysis of P0 Idua-W392X neonates injected ICV with C3.MPS LNPs. (B) NGS results at the expected site of base editing in three different sections of harvested brain tissue normalized to negative control (PBS); mean represented by horizontal line for each group (C) IDUA activity measured after harvest in three different sections of brain tissue from B6 mice (positive control), C3.MPS LNP-treated Idua-W392X mice (experimental group), and untreated Idua-W392X mice (negative control). (D) GAG amounts in three sections of brain tissue from B6, C3.MPS LNP-treated Idua-W392X, and untreated Idua-W392X mice. (E) Cytokine analysis in serum of Idua-W392X neonates treated 24 hours prior with C3.MPS LNPs. ** p < 0.01, *** p < 0.001, **** p < 0.0001 by two-way analysis of variance (ANOVA) with post-hoc Šídák’s multiple comparisons test; minimum n = 3 per treatment group; error bars represent SEM. (F) Serum anti-PEG IgM antibody levels in neonatal or adult mice one week following C3.MPS LNP treatment. *** p < 0.001 by Student’s t test with α = 0.05; minimum n = 3 per treatment group; error bars represent SEM. (G) NGS results at the Idua on-target site and the top computationally predicted off-target sites in brain genomic DNA of two C3.MPS LNP-treated Idua-W392X mice and one PBS-treated negative control. (H) Hematoxylin and eosin (H&E) stained whole brain tissue sections of PBS or C3.MPS treated Balb/c neonates with focus on the lateral ventricle. Scale bars: 1 mm (1X) and 50 μm (20x).
