Fig. 5 |. C3 LNP-mediated in utero mRNA delivery to the NHP brain and ex vivo performance in pediatric biological fluids and brain tissue.
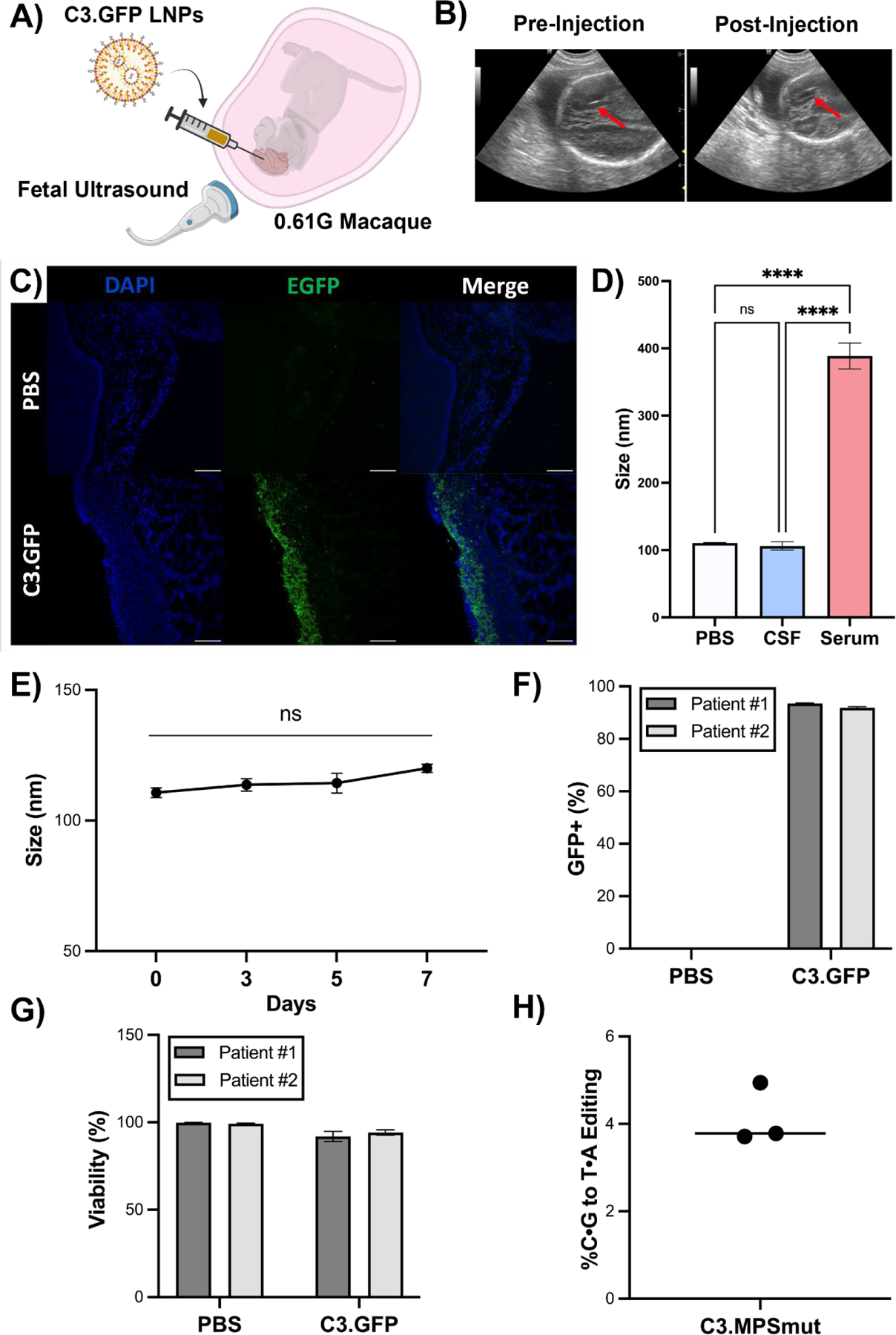
(A) Experimental scheme depicting ultrasound guided ICV injection of C3 LNP.GFP in a 0.61G Macaca fascicularis. (B) Ultrasound images (red arrow points to needle) pre- and post-ICV injection of C3.GFP LNPs in fetal macaque. (C) GFP immunohistochemistry on brain sections from C3 LNP.GFP-treated and negative control animals. Scale bars: 100 μm (10X). (D) Size and PDI measurements of C3.MPS LNPs incubated in PBS, human CSF, or human serum. **** p < 0.0001 by one-way ANOVA with Tukey’s multiple comparisons test. (E) Size measurements of C3.MPS LNPs incubated in human CSF over a 7-day time course. (F) GFP positivity in two patient-derived primary cell lines enriched for neurons after C3.GFP LNP treatment. (G) Viability after C3.GFP LNP treatment in both patient-derived cell lines. (H) NGS results in patient-derived precision cut brain slices treated with C3.MPS LNPs normalized to control (PBS). Outliers were detected using Grubbs’ test and removed from analysis; minimum n = 3 per treatment group; error bars represent SEM.
