Figure 3.
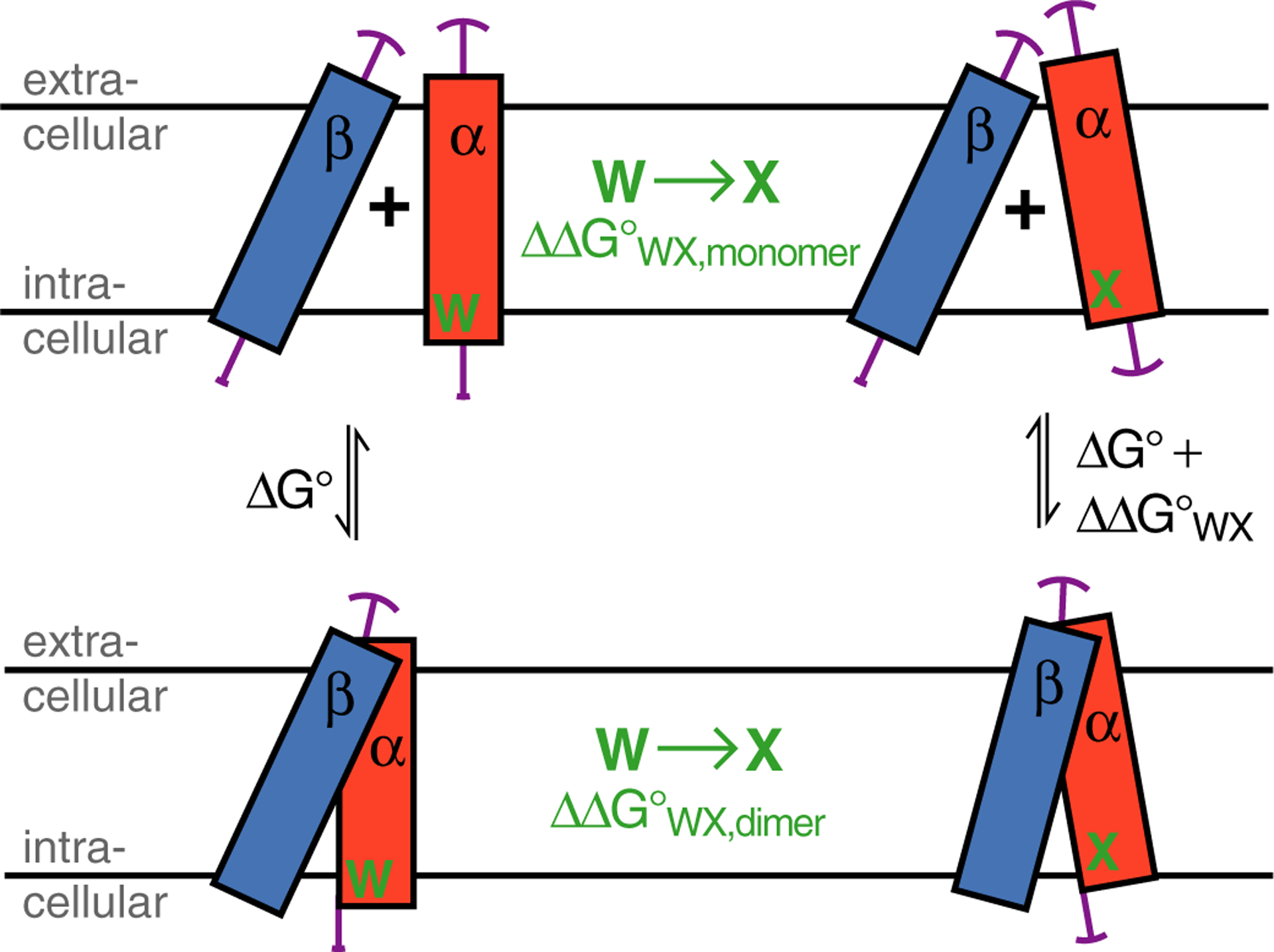
Illustration of the possible effects of Trp substitution on the folding of the integrin TM complex. A Trp substitution in integrin subunit α to residue X, termed WX, may alter its TM helix tilt and rotation, immersion depth and amplitudes of fluctuation around mean positions (illustrated by magenta arcs). The subsequent association with unperturbed subunit β is a compromise between ideal αβ helix-helix association and any required change to β TM helix orientation in order to reach the dimeric state. The αβ TM complex stability, termed ΔG°, changes upon WX substitution by ΔΔG°WX. Moreover, the effects of WX can also be seen in isolation for the monomeric and dimeric state, respectively, i.e., ΔΔG°WA,monomer underlies the transition from α to α(WX) orientations and ΔΔG°WX,dimer describes the change from αβ to α(WX)β orientations. Altogether, ΔG° + ΔΔG°WX,dimer = ΔΔG°WX,monomer + (ΔG° + ΔΔG°WX), which simplifies to ΔΔG°WX,dimer = ΔΔG°WX,monomer + ΔΔG°WX.
