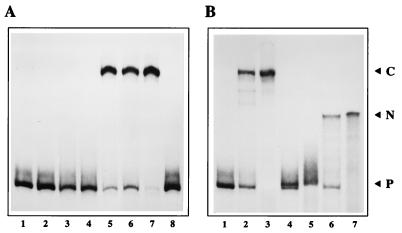
FIG. 3.
(A) NS1 can nick the right-hand origin only in the presence of a host protein. A 32P 3′-end-labeled right-hand hairpin origin probe in the flip orientation (lane 1) was incubated with 50 ng of NS1 alone (lane 2) or ∼2 μg of HeLa S100 extract alone (lane 3) in the presence of ATP. ATP was included in all reactions except that shown in lane 4, where probe, NS1, and HeLa extract were coincubated in its absence. HeLa extract was incubated with 50 ng of NS1 (lane 5), with 25 ng of NS1 (lane 6), or with 100 ng of NS1 (lane 7), and NS1 (50 ng) was incubated with partially purified PIF (lane 8). Reactions were terminated by heating to 60°C in 0.5% SDS, and the products were electrophoresed through 4.5% native acrylamide gels. (B) Structure of the nicked product. The 32P-labeled right-hand hairpin probe in the flip orientation (lane 1) was incubated with 50 ng of NS1 and ∼2 μg of HeLa S100 extract in the presence of ATP (lane 2). Reaction products equivalent to those shown in lane 2 were heated to 60°C in SDS and immunoprecipitated with a polyclonal antibody directed against the amino terminus of NS1 (lane 3). Total reaction products and anti-NS1 immunoprecipitates equivalent to those shown in lanes 2 and 3 were incubated with proteinase K (lanes 4 and 5, respectively) or incubated with proteinase K and then melted prior to electrophoresis (lanes 6 and 7, respectively). P, free probe; C, the nicked complex in which NS1 has become covalently attached to the probe at the nick site; N, the 32P-labeled, partially single-stranded product remaining after removal of both NS1 and the unlabeled duplex strand from the nicked hairpin complex.