FIGURE 2.
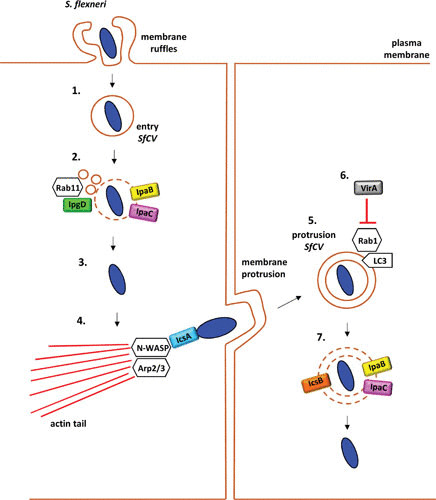
Schematic of the contribution of virulence factors to the intracellular lifecycle of S. flexneri (1). S. flexneri briefly resides within its entry vacuole (SfCV) (2). The SfCV is ruptured by the pore-forming activity of the T3SS translocon proteins IpaB and IpaC. IpgD facilitates vacuolar disruption by generating Rab11-macropinosomes that fuse to S. flexneri (3). Upon rupture of the SfCV, Shigella escapes into the host cytosol, from where the bacterium employs its IcsA to recruit actin cytoskeleton machinery, namely, N-WASP and Arp2/3 that polymerize actin filaments at one pole of the bacterium (4). Unidirectional actin polymerization propels the bacterium across the host cytosol, leading to protrusions that enable bacterial spread into the neighboring cell (5). In the secondary cell, S. flexneri is initially contained within a double-membrane vacuole (protrusion SfCV) (6). Recruitment of LC3 to the protrusion vacuole is controlled by the T3SS effector VirA, which targets the Rho GTPase Rab1 (7). IpaB, IpaC, and the T3SS effector IcsB promote bacterial escape from the protrusion of SfCV into the cytosol, enabling the bacterium to complete another infection cycle.
