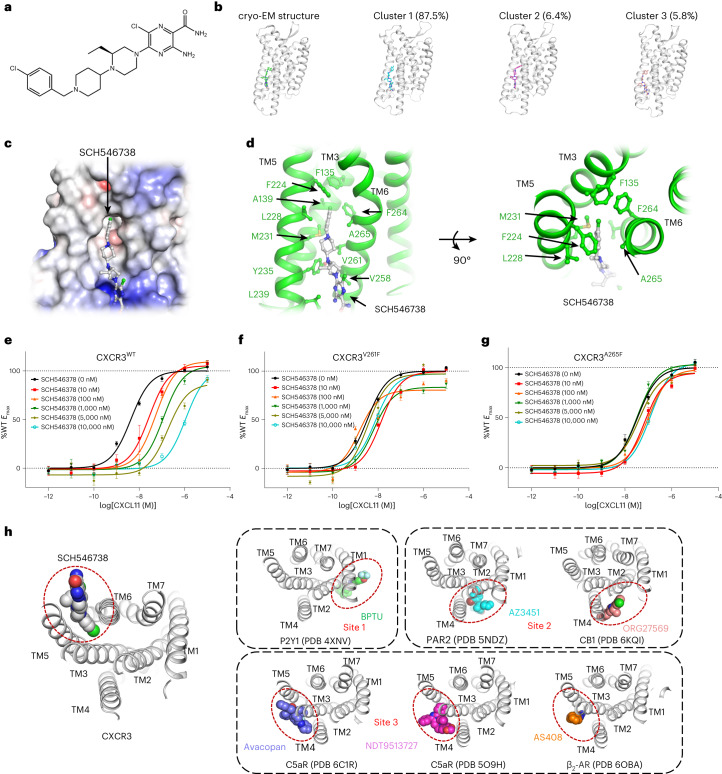
Fig. 6. Discovery of an allosteric site in the structure of CXCR3 occupied by SCH546738.
a, The chemical structure of SCH546738. b, Molecular dynamics simulation of SCH546738 binding. Shown are the cryo-EM structure and the top three cluster centroids. The size of each cluster is represented as the percentage of the total number of frames in the trajectories. c, A general view of the SCH546738 binding pocket in CXCR3. SCH546738 and CXCR3 are shown as a stick model (colored gray) and surface model (colored by electronic potential), respectively. d, Interactions between SCH546738 (gray) and CXCR3 (green). SCH546738 and the residues involved in interactions are shown as sticks. e, cAMP responses of CXCR3 to CXCL11 in the presence of SCH546738 at different concentrations. f, cAMP responses of CXCR3V261F to CXCL11 in the presence of SCH546738 at different concentrations. g, cAMP responses of CXCR3A265F to CXCL11 in the presence of SCH546738 at different concentrations. In e–g, the data represent means ± s.e.m. (n = 6 independent experiments). h, Comparison of the allosteric binding sites outside the TMs in class A GPCR. The receptors are shown as cartoons and the antagonists are shown as spheres. Allosteric binding sites are indicated by red circles.