Abstract
The concept of a ‘microbiota-gut-brain axis’ has recently emerged as an important player in the pathophysiology of Parkinson disease (PD), not least because of the reciprocal interaction between gut bacteria and medications. The gut microbiota can influence levodopa kinetics, and conversely, drugs administered for PD can influence gut microbiota composition. Through a two-step enzymatic pathway, gut microbes can decarboxylate levodopa to dopamine in the small intestine and then dehydroxylate it to m-tyramine, thus reducing availability. Inhibition of bacterial decarboxylation pathways could therefore represent a strategy to increase levodopa absorption. Other bacterial perturbations common in PD, such as small intestinal bacterial overgrowth and Helicobacter pylori infection, can also modulate levodopa metabolism, and eradication therapies may improve levodopa absorption. Interventions targeting the gut microbiota offer a novel opportunity to manage disabling motor complications and dopa-unresponsive symptoms. Mediterranean diet-induced changes in gut microbiota composition might improve a range of non-motor symptoms. Prebiotics can increase levels of short-chain fatty acid-producing bacteria and decrease pro-inflammatory species, with positive effects on clinical symptoms and levodopa kinetics. Different formulations of probiotics showed beneficial outcomes on constipation, with some of them improving dopamine levels; however, the most effective dosage and duration and long-term effects of these treatments remain unknown. Data from faecal microbiota transplantation studies are preliminary, but show encouraging trends towards improvement in both motor and non-motor outcomes.
This article summarises the most up-to-date knowledge in pharmacomicrobiomics in PD, and discusses how the manipulation of gut microbiota represents a potential new therapeutic avenue for PD.
Key Points
| Gut microbiota pathways influence levodopa absorption and contribute to side effects of dopaminergic medications used to treat Parkinson disease patients. |
| Dopaminergic medications can alter the composition of the gut microbiota. |
| Therapeutic interventions that target the gut microbiota have the potential to ameliorate levodopa pharmacokinetic parameters. |
| Several gut microbiota-targeted strategies are under evaluation as both symptomatic and disease-modifying therapies in Parkinson disease. |
Introduction
The discovery of a bidirectional communication between the brain and the gut, the so-called gut-brain axis, has revolutionized our current understanding of the physiology of the central nervous system (CNS) and the pathophysiology of several neurological conditions, including Parkinson disease (PD) [1]. Patients with PD are severely affected by gastrointestinal (GI) disorders throughout their lifetime and can present with GI symptoms (e.g., constipation) up to two decades before the onset of motor disturbances [2]. Pathological hallmarks of PD such as accumulation of abnormal α-synuclein [3] are detected in the enteric nervous system of PD patients before disease development and in individuals at high risk of developing PD such as those suffering from idiopathic REM (rapid eye movement) sleep behaviour disorder (iRBD) [4–7]. Recent multimodal imaging studies confirmed that some individuals with PD develop peripheral (cardiac and colonic) disease features prior to loss of dopaminergic putaminal uptake, reflecting a bottom-up ascension of α-synuclein pathology from the periphery to the CNS—the so-called ‘body-first’ subtype [8]. This in contrast to the ‘brain-first’ subtype characterised by initial pathological changes in the brain with subsequent spread to the brainstem and the periphery [8]. One of the most important routes connecting the gut and the brain, the vagus nerve, has been postulated to play a role in caudo-rostral spread of PD pathology, with truncal vagotomy appearing to protect against development of PD in both some epidemiological [9, 10] and in vivo [11] studies.
In the last two decades, increasing evidence has highlighted the crucial role played by the gut microbiota (GM) as regulator of the gut-brain axis, incorporating its contribution in the novel concept of ‘microbiota-gut-brain axis’ [1]. Multiple lines of evidence suggest that the microbiota-gut-brain axis can contribute to PD development and influence its progression, thus providing the rationale for GM manipulation as a therapeutic strategy in PD [12]. For instance, individuals with PD display increased colonic barrier permeability and intestinal inflammation [13–17], thus potentially allowing bacterial factors to enter the GI wall and trigger pathological changes implicated in PD. Several case-control studies demonstrated that dysbiosis characterises individuals with PD compared to healthy individuals, thus resulting in a pro-inflammatory milieu (reviewed in [18]). In vivo models showed that GM changes are sufficient to trigger PD-like pathological, neuroinflammatory and behavioural changes in mice, which can be reversed by restoring a ‘healthy’ GM [19–21]. In vitro studies suggested that bacterial components can trigger abnormal α-synuclein pathology in intestinal cells directly connected with the vagus nerve [22], supporting the hypothesis of α-synuclein accumulation triggered by GM changes.
In this article, we present an overview of the current knowledge regarding the interactions between GM and PD. In particular, we focus on the reciprocal effects of medications used to treat PD and GM composition, which is the research focus of the novel, cutting-edge subfield of pharmacogenomics called ‘pharmacomicrobiomics’ [23]. We then discuss possible therapeutic strategies to optimise drug absorption by modulating GM as well as the state-of-the-art evidence on therapeutic interventions targeting GM in PD.
Literature Search
We performed a literature search on 15 October 2023 for English-written articles published up to that date using the National Center for Biotechnology Information’s PubMed database (https://www.ncbi.nlm.nih.gov/pubmed) with the following search terms: ‘gut microbiota’ AND ‘Parkinson’ AND ‘therapies’ OR ‘levodopa’ OR ‘faecal microbiota transplantation’ OR ‘diet intervention’ OR ‘prebiotics’ OR ‘probiotics’, for the latter four using filters for clinical trial and randomised controlled trial (RCT). We selected the articles relevant to our article and included additional articles from their reference lists.
The Reciprocal Interaction Between Gut Microbiota (GM) and Pharmacological Therapies in Parkinson Disease (PD)
The initial symptomatic treatments of early motor PD rely on oral pharmacological therapies, such as levodopa, long-acting dopamine agonists, or monoamine oxidase type B inhibitors (MAO-BI). Although any of these options can be considered as first-line treatment, recent evidence-based guidelines published by the American Academy of Neurology recommend the use of levodopa for the treatment of early PD given its higher symptomatic efficacy [24].
In most PD patients, the response to dopaminergic medications becomes erratic and variable over time, leading to development of motor fluctuations, unpredictable or sub-optimal levodopa response, and ‘delayed on’ and ‘no on’ phenomena [25]. In this complex phase, the use of catechol-O-methyltransferase (COMT) inhibitors or MAO-BI can help. If not effective, non-oral therapies such as levodopa-carbidopa intestinal gel (LCIG) delivered continuously through a percutaneous endoscopic gastro-jejunal (PEG-J) tube, or deep brain stimulation, represent the alternative [24]. Albeit rarer, a sub-optimal clinical response to levodopa might also occur in some patients with PD (labelled as ‘primary non-responders’) [26].
Among the pathophysiological determinants of these disabling phenomena, the ability for the GM to directly influence drug bioavailability [27, 28] and determine side effects of dopaminergic medications [29], makes it a potential contributor. Unravelling the GM impact on drug metabolism is thus crucial not only for the purpose of optimising drug efficacy, but also for preventing sides effects in PD [29].
Bacterial Pathways Directly Implicated in Levodopa Metabolism
Levodopa, the mainstream treatment for PD, is a non-proteogenic large neutral amino acid (LNAA) produced by the hydroxylation at the meta-position of the phenyl ring of tyrosine [30]. Specific amino acid transporters transport levodopa at the GI level and blood-brain barrier (BBB). After passing by the BBB, levodopa is converted in the CNS into dopamine and 3-O-methyldopa (3-OMD) by the aromatic amino acid decarboxylase (AADC)-also known as DOPA decarboxylase (DDC), and the catechol-O-methyltransferase (COMT) enzymes, respectively [30].
Levodopa absorption and oral bioavailability are dependent upon several host-related factors. First, the absorption of levodopa is restricted to the proximal small intestine (duodenum and jejunum), where it is transported from the lumen into the bloodstream using a competitive transport system shared with other LNAAs [31]. Second, dietary proteins, converted into amino acids after digestion, inhibit levodopa absorption [32], and for this reason, low-protein diets can improve levodopa absorption [33]. Third, the enzymes AADC and COMT extensively convert levodopa to dopamine and 3-OMD also in the GI tract and bloodstream. Levodopa is therefore administered with inhibitors of AADC (carbidopa or benserazide) and COMT (tolcapone, entacapone and opicapone) to reduce its peripheral and GI metabolism [30, 34]. However, despite these inhibitors, up to 56% of levodopa fails to reach the brain [35], suggesting that other mechanisms influence levodopa metabolism.
Among these mechanisms, recent advances in pharmacomicrobiomics have shown the influence of the GM in regulating levodopa absorption. The first evidence that GM might influence levodopa bioavailability emerged in the 1970s. Urinary levels of m-tyramine, a by-product of dopamine dehydroxylation, were increased upon levodopa administration in six PD patients, but significantly decreased after antibiotic use, suggesting a role of GM in formation of m-tyramine from dopamine [36]. A few years later, another study demonstrated that m-hydroxyphenylacetic acid and m-hydroxyphenylpropionic acid were found in urine only of conventional rats fed with levodopa or dopamine, but not in germ-free rats, suggesting that dehydroxylation reactions were mediated by GM [37].
Despite these intriguing findings, the mechanisms by which GM metabolises levodopa in the small intestine have been clarified only in recent years. In 2019, van Kessel and colleagues showed that gut bacteria expressing tyrosine decarboxylases (TDCs), mainly species of genus Enterococcus (E. faecium and E. faecalis), could effectively decarboxylate levodopa to dopamine in the small intestine of rats [38]. Human AADC inhibitors did not inhibit levodopa decarboxylation activity in E. faecalis or E. faecium, and bacterial tyrosine decarboxylases (tdc) gene levels negatively correlated with plasma and proximal jejunal levels of levodopa/carbidopa in rats fed with levodopa/carbidopa, suggesting that higher abundance of gut bacteria encoding for tdc gene in the small intestine reduced levodopa/carbidopa absorption [38]. These conclusions were corroborated by findings in PD patients on levodopa therapy where bacterial tdc gene relative abundance positively correlated with the daily dose of levodopa and disease duration [38]. In a recent cross-sectional study conducted on PD patients, it was observed that moderate responders to levodopa had a higher abundance of tdc gene and E. faecalis than good responders [39]. The abundance of bacterial tdc gene rather than E. faecalis was independently associated with levodopa responsiveness, possibly because tdc gene also exists in other bacterial species such as E. faecium and Lactobacillus brevis, so bacterial gene abundance might explain drug metabolism better than species abundance [39]. Interestingly, clinical features (disease duration or severity) and PD medication use were not associated with tdc gene abundance, supporting the potential use of relative abundance of bacterial tdc gene as a biomarker of levodopa responsiveness before treatment initiation [39].
Maini Rekdal and colleagues identified that the bacterium responsible for dehydroxylation of dopamine into m-tyramine is a strain of Eggerthella lenta [35], which was previously shown to be involved in drug metabolism [40]. The same authors found that the L-tyrosine analogue (S)-α-fluoromethyltyrosine (AFMT), a nontoxic selective inhibitor of TDC (but not AADC), inhibited gut microbial levodopa decarboxylation in vitro. Combined administration of AFMT with levodopa/carbidopa to mice colonized with E. faecalis increased the peak serum concentration of levodopa compared to vehicle, opening up the possibility of developing levodopa therapies targeting both host and gut microbial decarboxylation [35].
Other than in levodopa absorption, the GM may play an important role in determining side-effect profiles in levodopa-treated PD patients. Deamination of levodopa by Clostridium sporogens can produce the metabolite 3-(3,4-dihydroxyphenyl)propionic acid (DHPPA), which inhibited muscle contraction in the ileum in an ex vivo model [29]. This metabolite (DHPPA) was significantly higher in faecal samples from PD patients on levodopa than age-matched healthy controls, and was actively produced by the GM in PD patients’ faecal suspensions, supporting a possible implication of GM in levodopa-induced side effects [29].
Other Bacterial Mechanisms Influencing Levodopa Bioavailability
Small intestinal bacterial overgrowth (SIBO), a condition characterised by an increased concentration of bacteria above 105 colony-forming units/ml and/or presence of colonic-type bacteria in the small intestine [41, 42], is reported in up to 54% of PD patients, and associates with development of motor complications and variability in motor response [43–45]. SIBO could influence levodopa metabolism because it can select gut bacteria expressing tdc gene in PD patients [30], or alternatively increase small intestine permeability and inflammation [43]. Regardless of the mechanisms, if SIBO directly interferes with levodopa absorption, eradication of SIBO should improve levodopa bioavailability and motor symptoms. In an open-label trial, 400 mg rifaximin administered three times a day for 1 week was tested in a group of 18 SIBO-positive PD patients [43]. One month after initiation of antibiotic treatment, nearly 80% of patients were SIBO-negative, with improvement in motor fluctuations (both OFF time and delayed-ON), but there were no significant differences in levodopa pharmacokinetic parameters. Unfortunately, the study reported a high percentage of relapse after 6 months from SIBO eradication (42.9%) [43]. Another single-centre, double-blind, RCT was designed to evaluate the effect of rifaximin on OFF symptoms in SIBO-positive PD patients with at least 4 h/day of ‘OFF’ time (ClinicalTrials.gov ID: NCT02470780, SIBO-PD). The study failed because of difficulties in patient recruitment and unexplained spontaneous conversion to SIBO negativity in patients in the placebo arm [46]. Taken together, these results pose some questions about the role played by SIBO in levodopa metabolism.
Another bacterium that could interfere with levodopa absorption is Helicobacter pylori (HP). HP infection is associated with increased PD risk [47], and progressive deterioration of motor symptoms [48, 49]. In initial studies, HP-positive PD patients displayed more severe motor fluctuations compared to HP-negative patients, and eradication of HP by antibiotic treatment (amoxicillin+clarithromycin) improved motor fluctuations and significantly increased the area under the curve (AUC) of levodopa plasma concentration by about 20% in these individuals [50, 51]. The benefits on motor fluctuations following HP eradication were confirmed by follow-up studies [52–54]. However, a small double-blind RCT (N = 67) showed no improvement in motor function tested in the ON phase, at either short-term (12-week) or longer-term (52-week) follow-up [55]. Moreover, no significant differences in pharmacokinetic parameters of levodopa and 3-OMD were found between HP-positive and -negative PD patients in another study [56]. Although the small sample size of the more recent studies could have reduced their statistical power, current data regarding benefits of HP eradication remain controversial. Prior to the design of larger clinical trials evaluating the potential benefit of HP eradication in PD, further research aiming to disentangle the mechanisms behind levodopa absorption modulation by HP infection is needed. Only a few mechanisms have been proposed so far. Reduced gastric acidity (hypochlorhydria), which is linked to HP infection, can favour the development of SIBO, whose potential role as modulator of levodopa metabolism has been discussed above [57]. The existence of a direct interaction between levodopa and the outer membrane proteins of HP was suggested by one in vitro study where levodopa concentration was reduced in a time- and HP density-dependent manner when exposed to HP, and pre-incubation of levodopa with HP showed significantly reduced bacterial adhesion to gastric epithelial cells [58]. Further studies are therefore needed to clarify whether the benefits achieved by HP are exclusively secondary to HP eradication or can be due to elimination of other bacterial species that might interfere with levodopa absorption in the small intestine [30].
Indirect evidence of GM influence on levodopa absorption comes from other examples of effective use of antibiotics at reducing motor fluctuations and dyskinesias in PD (beyond SIBO or HP eradication) [59]. Treatment with sodium phosphate enema followed by oral rifaximin and polyethylene glycol for 7 and 10 days improved duration and severity of dyskinesia in 57% of cases, and functional impact and complexity of motor fluctuations [59]. Levodopa plasma levels were not measured pre- and post-intervention, so more research is needed to clarify the effects of such treatment on levodopa pharmacokinetics.
To conclude, a variety of bacterial pathways are implicated in the metabolism of levodopa, expanding the range of potential pharmacological targets to improve levodopa absorption. These might include, among others, the use of bacterial TDC inhibitors and the selective eradication of HP. A summary of possible GM-focused strategies to screen response to treatment in drug-naïve PD patients and optimise levodopa absorption in treated patients is presented in Fig. 1. Further studies are needed to better understand this complex interaction between GM, host and medications in PD. Although this goes beyond the scope of this review, it is worth mentioning that other factors can contribute to limit levodopa absorption in PD patients. These include transport barriers, such as dysphagia, delayed gastric emptying, and slow transit constipation. Therapeutic strategies aimed to address these disturbances can therefore improve levodopa bioavailability [25].
Fig. 1.
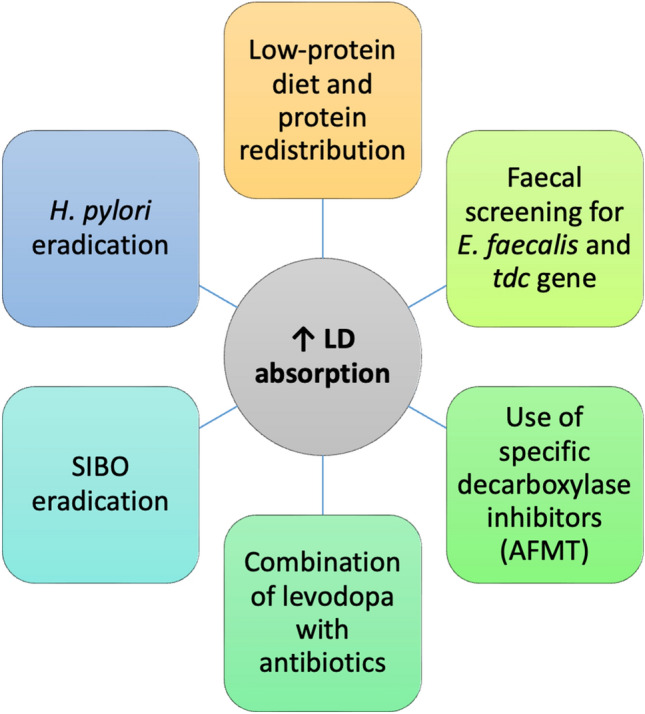
Possible gut microbiota (GM)-based strategies to optimise levodopa absorption. AFMT (S)-α-fluoromethyltyrosine, LD levodopa, SIBO small intestinal bacterial overgrowth, tdc tyrosine decarboxylase
PD Medications Influence GM Composition
The interaction between GM and medications is bidirectional, with a direct effect of pharmacological treatments used in PD on GM composition. This observation is important to identify disease-related changes in GM of PD patients, not secondary to drugs, and for the potential interference of these changes on bioavailability of concomitant medications and overall gastrointestinal health. Although this is beyond the primary scope of this article, we will briefly summarise the evidence on GM changes induced by use of PD medications. For a comprehensive review on the effect of non-pharmacological therapies, such as deep brain stimulation, on GM, the reader is referred elsewhere [27].
Levodopa effects were evaluated in a small group of PD patients (N = 19) after 90 days from treatment initiation. Levodopa did not induce any alteration in α-diversity (i.e., diversity within a community sample) or β-diversity (i.e., similarity between two community samples), or relative abundance of bacterial genera; only a marginal lower abundance of bacteria belonging to Clostridium group IV was found in those patients who showed a better response to levodopa [60]. Significant alterations in the abundance of family Bacillaceae [61], increased relative abundance of genera Peptoniphilus and Finegoldia, and decreased relative abundance of genus Faecalibacterium and Ruminococcus gauvreauii were detected in PD patients treated with levodopa versus controls; however, no significant differences at the genus or family levels were found between PD patients on levodopa and levodopa-naïve patients [62]. In another study evaluating 46 patients treated with levodopa, decreased abundance of genera Faecalibacterium, Roseburia and Pseudobutyrivibrio, and species belonging to Bacteroides, Blautia and Lachnospira, and increased abundance of Colinsella, were found in comparison to drug-naïve subjects [63].
We mentioned above that LCIG represents an effective strategy to address motor fluctuations in advanced cases of PD [64]. After 4 weeks from LCIG initiation, patients displayed an over-representation of Pseudoflavonifractor and Escherichia/Shigella, and under-representation of Gemmiger [65]. Considering that Escherichia/Shigella are bacteria that tolerate acidic conditions [66], the authors suggested that their higher abundance was secondary to the mildly acidic (~ pH 6.0) properties of LCIG [65]. In another study, increased abundance of Proteobacteria and Enterobacteriaceae and reduction of Firmicutes, Lachnospiraceae and Blautia were detected in LCIG patients compared to drug-naïve PD patients [63]. When patients treated with oral levodopa and LCIG were compared, there was a significant difference in β-diversity between groups and higher abundance of genera Escherichia and Serratia in LCIG patients [63]. Intestinal bacteria were collected from the tips of the PEG-J tube in six patients receiving LCIG therapy after tube replacement. E. faecalis was identified in four out of the six patients, and tdc in two patients out of these four. Only the four E. faecalis positive samples showed the ability to metabolize levodopa to dopamine in vitro [67]. Regardless of the presence of E. faecalis, however, no differences in mean blood concentration of levodopa were detected among patients. Despite the limited sample size, these preliminary findings might suggest that the effect of GM in metabolising levodopa in patients receiving LCIG therapy is blunted, possibly due to the shorter time of the drug in the GI tract compared to oral administration of levodopa [67].
Use of COMT inhibitors, especially entacapone, was associated with reduction in abundance of Firmicutes, species Faecalibacterium prausnitzii [68, 69] and Lachnospiraceae [68, 70], and increased abundance of Actinobacteria, Lactobacillaceae, Porphyromonadaceae and Proteobacteria [68]. Use of opicapone or tolcapone was not associated with changes in F. prausnitzii [71]. Entacapone use was uniquely associated with reduced relative abundance of Lactobacillus, Intestinibacter, Dorea and Blautia, and increased abundance of Christensenellaceae_R_7_group, Eubacterium and Bifidobacterium [72], and negatively correlated with relative concentration of butyrate, one of the most abundant short-chain fatty acids (SCFAs) produced by GM [69, 71]. Considering that SCFAs play a physiologically central role in several host functions and serve as essential energy sources for colonocytes [1], the potential implications of entacapone on GI physiology deserve attention.
The impact of dopamine agonists in combination with levodopa-carbidopa on GM was evaluated in rats. Treatment with pramipexole and ropinirole (in combination with levodopa-carbidopa) for 14 days significantly reduced small intestinal motility, increased bacterial overgrowth in the distal small intestine, increased bacterial richness in the jejunum (not the ileum), and had a significant effect on β-diversity in both proximal small intestine and ileum, with Muribaculaceae and Lactobacillus mostly contributing to the variation [73]. Alterations in the abundance of specific taxa were found (for instance, decreased abundance in Romboutsia spp. in the proximal small intestine and Lachnospiraceae spp. in the ileum in the pramipexole-treated group, and increased Lactobacillus spp. in both the pramipexole- and ropinirole-treated groups) [73]. Interestingly, there was a negative correlation between Lactobacillus abundance and plasma levodopa levels [73]. Although we acknowledge that there are differences in the composition of the GM between rats and humans and therefore translating findings requires caution, it is interesting to note that the above-mentioned changes in Lactobacillus or Lachnospiraceae observed in the healthy rats treated with dopamine agonists and levodopa have been previously reported in PD patients when compared to healthy subjects [18]. Increasing doses of levodopa were found to correlate with reduced levels of SCFA-producing bacteria (e.g., Lachnospiraceae) and increased levels of Lactobacillus or Bifidobacterium, supporting the hypothesis that these alterations in the GM composition could be a consequence of PD medications, rather than of the disease itself [74]. Deciphering the exact role played by single medications (levodopa-carbidopa vs. dopamine agonists) on the GM composition and their effect on gastrointestinal motility remains an open but crucial area to address.
To date, a few longitudinal studies have investigated the impact of medications on GM composition over time. Entacapone and dopamine agonists positively contributed to an increase in tdc gene abundance over 2-year follow-up, whereas the opposite effect was achieved by MAO-BI [75]. When PD patients were separated into slow- and rapid-progressing clinical sub-categories, specific medications contributed to changes in tdc abundance, such as entacapone in rapid-progressing PD [75]. Regarding LCIG, the same authors who evaluated the short-term effect of LCIG also investigated long-term effects. After 12 months from LCIG initiation, inconsistent results compared to short-term changes were found, with relative increased abundance of family Prevotellaceae and genera Prevotella and Bacillus, and relative reduced abundance of genera Hespellia, Eggerthella, Holdemania, Gordonibacter and Acetanaerobacterium [76].
Overall, the current results might suggest that the GM composition in PD patients is not static, and specific drugs and treatment duration might differentially shape the GM composition. The small sample size of most studies and the use of low-taxonomical sequencing techniques, for example, 16S rRNA amplicon sequencing, currently limit our ability to draw definite conclusions. Large, prospective studies on human cohorts, in combination with in vivo models, are needed to elucidate which changes occur as a consequence of the disease itself and which are caused by medications. Moreover, future studies will need to evaluate the effect of such changes on other drugs pharmacokinetics and disease progression.
GM-Focused Interventions in the Treatment of PD
In the previous section, the evidence supporting the role of GM in influencing levodopa absorption, and some possible therapeutic options to ameliorate levodopa metabolism, were presented. In addition, several GM-focused strategies have been tested in PD with the purpose of ameliorating symptoms and/or slowing down disease progression. These include dietary interventions, prebiotic fibres, probiotics and faecal microbiota transplantation [77].
In the following section, we present the current evidence related to the application of GM-focused strategies in human PD populations. We focus on studies designed in a randomised, controlled fashion that have incorporated evaluation of GM composition pre- and post-intervention.
Dietary Interventions
Diet modifications can reduce risk of PD development as well as attenuate symptoms, but whether the effects of these interventions are mediated by changes in GM or alterations in chronic inflammatory status is unclear [77]. Aside from a few exceptions, most studies evaluating dietary interventions did not include GM analysis in their study design. An overview of dietary and/or supplement interventions tested in RCT studies is summarised in Table 1 [33, 78–89].
Table 1.
Overview of relevant randomised controlled trials testing dietary and/or supplement interventions in Parkinson disease
| Diet or supplement interventions | RCT study design | No. of individuals included in final analyses | Treatment duration | Main outcome | References |
|---|---|---|---|---|---|
| Controlled protein diet with consumption of low-protein products vs. balanced diet | Cross-over, single-blind | 18 | 8 weeks | Reduced OFF time | Barichella (2006) [33] |
| Fish oil containing omega-3 vs. placebo | Double-blind | 29 | 12 weeks | Reduced depressive symptoms and/or remission of depression | da Silva (2008) [81] |
| Vitamin B supplementation (B6, B12, folic acid) vs exercise vs combined vitamin + exercise vs. no intervention | Not specified | 36 divided into 4 groups | 6 weeks |
Reduced homocysteine levels (vitamin B supplementation group). Increased glutathione levels, strength and aerobic capacity (exercise group). No greater changes in vitamin and exercise group vs. single interventions |
DiFrancisco-Donoghue (2012) [82] |
| Individualised dietetic advice vs. standard care | Not specified | 10 (intervention) vs. 9 (standard care) | 12 weeks | No changes in quality of life | Sheard (2014) [87] |
| Amino acid supplementation vs. placebo | Double-blind | 7 (intervention) vs. 7 (placebo) | 6 months |
No changes in symptoms or medications requirement. Improvement in insulin sensitivity and reduction of oxidized glutathione levels in treated arm |
Cucca (2015) [80] |
| Whey protein supplementation vs. soy protein (control) | Double-blind | 15 (intervention) vs. 17 (control) | 6 months |
No changes in motor function. Increased plasma levels of reduced glutathione and ratio of reduced to oxidized glutathione, and reduced homocysteine levels in treated arm. Increased plasma BCAA and EAA in treated arm |
Tosukhowong (2016) [89] |
| 1,000 mg omega-3 fatty acids from flaxseed oil plus 400 IU vitamin E supplements vs. placebo | Double-blind | 30 (intervention) vs. 30 (placebo) | 12 weeks | Improvement in MDS-UPDRS score, decrease in high-sensitivity C-reactive protein and insulin, and increase in total antioxidant capacity in treated arm | Taghizadeh (2017) [88] |
| Low-fat diet vs. ketogenic diet | Single-blind | 20 (low-fat) vs. 18 (ketogenic) | 8 weeks | Decreased MDS-UPDRS scores in both groups, with more decreased MDS-UPDRS part I scores in ketogenic group | Philips (2018) [85] |
| Whey protein-based nutritional supplement + leucine + vitamin D vs. standard hospital diet | Single-blind | 75 (intervention) vs. 75 (control) | 30 days multidisciplinary intensive rehabilitation treatment | Greater increase in distance walked during 6-min walking test in treated arm | Barichella (2019) [78] |
| Mediterranean diet vs. normal diet | Single-blind | 35 (intervention) vs. 35 (control) | 10 weeks | Improvement in mean score of MOCA test and sub-scores (executive function, language, attention, concentration, and working memory) in treated arm | Paknahad (2020) [83] |
| Mediterranean diet vs. normal diet | Single-blind | 36 (intervention) vs. 34 (control) | 10 weeks |
Improvement in MDS-UPDRS score. Increased serum total antioxidant capacity in treated arm |
Paknahad (2022) [84] |
| High flavonoid cocoa beverage vs. low (control) | Double-blind | 15 (high) vs. 15 (low) | 6 days | Small effect on fatigability in treated arm | Coe (2022) [79] |
| Mediterranean diet vs. standard care | Single-blind | 46 (not specified by group) | 8 weeks | Changes in constipation at 10 weeks | Rush (2021) [86] |
BCAA branched-chain amino acids, EAA essential amino acids, MDS-UPDRS Movement Disorders Society Unified Parkinson’s Disease Rating Scale, MOCA Montreal Cognitive Assessment, RCT randomised controlled trial
The most studied dietary intervention in PD is the Mediterranean diet. The Mediterranean diet is associated with a 25% risk reduction in PD development, suggesting a neuroprotective effect that might be mediated by GM [90]. A single-arm, 5-week Mediterranean diet intervention study conducted in eight people with PD in the USA resulted in increased adherence to the Mediterranean diet, weight loss and improvement in constipation, with increased abundance of Roseburia and decreased abundance of Bilophila post-intervention [91]. Following this study, the MEDI-PD study was designed (ClinicalTrials.gov ID: NCT04683900): this was an RCT, double-blind study in which participants were randomised to follow either a Mediterranean diet or their habitual diet for 8 weeks [86]. Primary outcome was change in constipation at 10 weeks compared to baseline [86]. Recruitment status is completed, and results are yet to be published. In two RCT studies, the Mediterranean diet improved cognitive function and global disease severity, and promoted antioxidant benefits in the intervention group [83, 84].
The ketogenic diet, characterised by high-fat, low-carbohydrate and adequate protein intake [92], has been marginally evaluated in PD. An initial open-label study conducted on five patients who adhered to a ketogenic diet for 28 days showed improvement in disease severity as measured by Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) in all subjects (decrease in total scores varied from 21% to 81%) [93]. Only one RCT study has been completed where patients were randomized to either follow a low-fat or ketogenic diet for 8 weeks. Amelioration of disease severity was observed in both groups; however, the ketogenic group showed more significant improvement in non-motor symptoms as measured by MDS-UPDRS part I [85].
The combination of dietary intervention (ovo-lacto vegetarian diet intervention including SCFAs for 14 days) and bowel cleansing (faecal enema for 8 days) was evaluated in a small group (N = 10) of PD patients [94]. At 1-year follow-up, combined treatment improved motor function and reduced medication requirements [94]. No long-term evaluation of the GM was performed in this study [94], so whether the observed clinical benefits can be ascribed to changes in the GM composition remains to be proven.
A number of different supplementation interventions have been tested in RCTs in PD, but no definite conclusions can be drawn from most of these studies (see Table 1). Although some resulted in a positive metabolic effect induced by the intervention, no beneficial clinical effect was detected [80, 89]. The main limitation of these studies might be the small sample size, which is one of the commonest challenges in dietary clinical trials [95]. Moreover, the evaluation of GM composition was absent in their study design.
A small exploratory, open-label pilot study was launched in 2022 to assess the SCFA-prodrug tributyrin as a potential therapy for PD (ClinicalTrials.gov ID: NCT05446168; BUTTER study). Unfortunately, the study was terminated in late 2023 because of funding withdrawal after recruiting only 18 patients.
Convincing results were reported only from a relatively large RCT study recruiting hospitalised patients with PD or other parkinsonian disorders undergoing a 30-day multidisciplinary intensive rehabilitation treatment (MIRT). Patients were randomised 1:1 to receive either a whey protein-based nutritional supplement enriched with leucine and vitamin D or a standard diet (N = 75/group). The muscle-targeted nutritional supplement significantly improved the efficacy of MIRT gait outcomes and helped the recovery of lower body physical function and the preservation of muscle mass compared to standard diet, supporting the use of muscle-targeted nutritional support in addition to intensive rehabilitation programmes in PD patients [78].
Prebiotics
Prebiotics are currently defined as “substrates that are selectively utilised by host microorganisms conferring a health benefit” [96]. The source of these dietary fibres can vary, from unrefined wheat and barley, soybeans, breast milk and raw oats, to non-digestible carbohydrates and oligosaccharides, including galacto-oligosaccharides (GOS), fructo-oligosaccharides (FOS), inulin and lactulose [77, 97]. Consumption of prebiotic fibres can favour the growth of bacterial families such as Ruminococcaceae and Bacteroidaceae that produce SCFA [98, 99]. Recent in vitro fermentation studies demonstrated that when exposed to different prebiotic fibres, faeces from PD patients can produce SCFA at a similar level to controls, indicating the potential of prebiotic fibres to increase SCFA in PD patients [100]. Some studies have investigated the use of prebiotics in PD patients.
Two studies evaluated the effect of 8-week treatment with diet rich in either insoluble fibre (DRIF, composed of wheat, pectin and dimethylpolyoxyhexane-900) [101] or psyllium [102] in a small number of PD patients (N = 19, and N = 7, respectively). Positive outcomes were reported in both studies, with improvement in motor function and constipation and increase in plasma levodopa levels after DRIF [101], and increase in stool frequency and weight but no effect on colonic transit time after psyllium treatment [102].
More recently, the effect of 8-week dietary supplementation with resistant starch (RS; 5 g RS given twice a day orally) compared to solely dietary instructions (high-fibre diet) was evaluated in a group of 57 PD patients (of whom 32 received RS and 25 dietary instructions only) and 30 control subjects receiving RS (the RESISTA-PD trial, ClinicalTrials.gov ID: NCT02784145) [103]. RS supplementation significantly increased faecal butyrate and reduced faecal calprotectin concentrations in the PD group compared to baseline, whereas no changes were observed in controls supplemented with RS or in PD patients given dietary advice only [103]. Non-motor symptoms and depression, but not bowel habits, improved after RS supplementation in PD patients. At the GM level, the ratio between relative abundances of Dorea longicatena and Blautia wexlerae to Ruthenibacterium lactatiformans was positively associated with relative butyrate concentrations [103].
Another open-label, non-randomized study (ClinicalTrials.gov ID: NCT04512599) was conducted in newly diagnosed, non-medicated (N = 10) and treated (N = 10) PD patients to evaluate the effect of a 10-day prebiotic intervention [100]. The intervention was well tolerated and safe, reduced the relative abundance of putative pro-inflammatory bacteria (e.g., phylum Proteobacteria), and increased the abundance of putative SCFA-producing bacteria (e.g., species Fusicatenibacter saccharivorans, Parabacteroides merdae) in faeces with an increase in plasma levels of SCFA [100]. Moreover, the prebiotics improved markers of intestinal barrier integrity and inflammation (like plasma zonulin levels), and decreased levels of neurofilament light chain as a marker of neurodegeneration [100]. The authors hypothesized that the latter change might be mediated via changes in plasma levels of SCFA [100]. From a clinical perspective, gastrointestinal symptoms improved in treated PD participants, and motor function improved in all patients, but the unblinded nature of the study poses limitations to the interpretation of these findings [100].
Probiotics
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [104]. The most common bacteria used as probiotics, Lactobacillus and Bifidobacterium, have potential benefits in the restoration of favourable GM, thus promoting healthy gastrointestinal tract and immune system [104]. Use of probiotics has been successfully applied in several in vitro and in vivo studies (for a reference, the reader is directed elsewhere [77]), and initial studies in human populations showed beneficial effects on constipated PD individuals treated with probiotic Lactobacillus casei strain Shirota [105]. Based on these premises, numerous probiotics preparations have been tested in the controlled setting of RCTs. An overview of the study design, outcome and GM changes of the most important RCTs is presented in Table 2 [106–113].
Table 2.
Overview of randomised clinical trials investigating probiotics supplementation in Parkinson disease (adapted from [77])
| Probiotic type | No. of individuals included in final analyses (probiotics vs. placebo) | Treatment duration | Clinical outcome | Influence on gut microbiota | Other effects | References |
|---|---|---|---|---|---|---|
| Fermented milk, containing multiple probiotic strains and prebiotic fibre | 80 vs. 40 | 4 weeks |
Increase in number of complete weekly bowel movements Changes in stool consistency, reduction in laxatives use; no change in dopaminergic therapy |
NA | NA | Barichella (2016) [106] |
| Lactobacillus acidophilus and Bifidobacterium infantis | 20 (probiotics) vs. 20 (receiving trimebutine) | 12 weeks |
Probiotics group: reduction of abdominal pain and bloating Trimebutine group: reduction of abdominal pain, bloating and incomplete defecation |
NA | NA | Georgescu (2016) [108] |
| Lactobacillus acidophilus, Bifidobacterium bifidum, Lactobacillus reuteri, Lactobacillus fermentum | 30 vs. 30 | 12 weeks | Reduction in MDS-UPDRS total score | NA |
Reduced high-sensitivity C-reactive protein and malondialdehyde, and increased glutathione levels Reduced insulin levels and insulin resistance, and increased insulin sensitivity Trend towards decrease in triglycerides and VLDL-cholesterol levels |
Tamtaji (2019) [111] |
|
Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus lactis, Bifidobacterium infantis, Bifidobacterium longum (Hexbio®) |
22 vs. 26 | 8 weeks |
Improvement in number of bowel movements and gut transit time. No differences in motor outcomes, non-motor symptoms and quality of life |
NA | NA | Ibrahim (2020) [109] |
| Lactobacillus acidophilus, Lactobacillus gasseri, Lactobacillus reuteri, Lactobacillus rhamnosus, Bifidobacterium bifidum, Bifidobacterium longum, Enterococcus faecalis, Enterococcus faecium | 34 vs. 38 | 4 weeks | Improvement of spontaneous bowel movements, stool consistency and quality of life related to constipation | NA | No changes in faecal calprotectin | Tan (2021) [112] |
| Bacillus licheniformis, Lactobacillus acidophilus, Bifidobacterium longum, Enterococcus faecalis | 23 vs. 23 | 12 weeks | Improvement of complete weekly bowel movements and defecation effort | Increased abundance of Negativicutes and decreased abundance of Prevotellaceae after treatment | NA | Du (2022) [107] |
| Bifidobacterium animalis subsp. lactis (Probio-M8) (added to benserazide and dopamine agonist) | 48 vs. 34 | 12 weeks |
Improvement of sleep quality, anxiety and gastrointestinal symptoms Higher scores for satisfaction of treatment and possibility of continuing medication in probiotic group vs. placebo |
Increased abundance of Bifidobacterium animalis, Ruminococcaceae and Lachnospira, and reduced abundance of Lactobacillus fermentum and Klebsiella oxytoca in treated group vs. placebo | Increased acetate, dopamine and reduced glutamine and tryptophan serum concentration in probiotic group | Sun (2022) [110] |
| Lacticaseibacillus (formerly Lactobacillus) paracasei strain Shirota | 65 vs. 63 | 12 weeks | Improvement of constipation, non-motor symptoms and quality of life |
No difference in β-diversity between baseline and follow-up Change in relative abundance of genus Lacticaseibacillus in probiotic group compared to baseline and placebo group |
Decreased faecal concentration and increased plasma concentration of L-tyrosine in probiotic group | Yang (2023) [113] |
CFU colony-forming unit, MDS-UPDRS Movement Disorders Society Unified Parkinson’s Disease Rating Scale, NA not available, RCT randomized controlled trial, VLDL very-low-density lipoproteins
Improvement in constipation symptoms was the primary outcome in most studies, and all the studies reported a significant improvement in the treated arm [106, 109, 110, 112, 113]. Global disease severity measured by MDS-UPDRS significantly improved in one study [111], whereas severity of motor function measured by MDS-UPDRS part III was significantly reduced in the treated arm at 1 and 3 months versus baseline, while only at 3 months in the placebo arm in another study [110]. One study compared probiotics to trimebutine, a prokinetic agent, instead of placebo. Interestingly, both treatments effectively addressed bloating and abdominal pain, whereas only trimebutine ameliorated incomplete defecation [108]. The authors concluded that the association of prokinetics with probiotics could be beneficial in some individuals with PD [108]. In one RCT study, the use of multi-strain probiotics downregulated gene expression of interleukin-1 (IL-1), IL-8 and tumour necrosis factor α (TNF-α), whereas upregulated transforming growth factor beta (TGF-β) and peroxisome proliferator activated receptor gamma (PPAR-γ) in peripheral blood mononuclear cells (PBMC) of PD patients [114].
Only three studies reported GM data pre- and post-intervention [107, 110, 113]. Interestingly, one study reported increased serum levels of acetate and dopamine after the Probio-M8 (Bifidobacterium animalis subsp. lactis) supplementation [110]. Another study showed that probiotic Lactobacillus paracasei strain Shirota was able to change concentration levels of L-tyrosine in stool (reduction) and plasma (increase), with a negative correlation between plasma and stool concentrations [113]. Both studies seem to point towards an effect of probiotics supplementation on dopaminergic levels.
Based on the available studies, the latest recommendations by the MDS EBM Committee published in 2019 considered probiotics and prebiotic fibres as efficacious, clinically useful and associated with an acceptable risk profile that does not require specialized monitoring in clinical practice [115]. Nonetheless, numerous questions remain. First, more studies including metagenomics and metabolomics analysis on faecal samples are needed to understand the causal relationship between clinical outcomes and treatments. Second, type and duration of treatment varied across studies, and more research is needed to refine the best regimen, dose and duration. Third, the studies conducted so far evaluated the short-term effect of intervention, so long-term evaluations are needed. Fourth, it is very likely that not all individuals with PD might benefit from these treatments to the same extent, so screening tools based, for instance, on GM profile should be applied to stratify individuals a priori and personalise treatments.
Faecal Microbiota Transplantation
Faecal microbiota transplantation (FMT) consists of transplantation of filtered faecal material from healthy donors into the recipient’s gut [77]. The beneficial effects of FMT from healthy donors have been clearly shown in several PD animal models where behavioural, motor, pathological and GM features were rescued by the treatment [20, 21, 116]. These promising results together with the already broad application of FMT in humans to treat recurrent Clostridium difficile infection [117] have prompted various groups of researchers to test the efficacy of FMT in PD populations.
Two preliminary studies conducted on a small group of PD patients evaluated the effect of FMT in humans [118, 119]. In one study conducted on 11 PD patients with constipation, 12 weeks after FMT administered via nasoduodenal tube, both motor and non-motor symptoms, especially constipation, improved. Moreover, FMT increased the relative abundance of Blautia and Prevotella, reduced the relative abundance of Bacteroidetes, and corrected SIBO [118]. In the other study, 15 patients with PD received FMT (ten via coloscopy and five via nasal-jejunal tube) with improvement in sleep, mood, non-motor and motor symptoms at 1 and 3 months after treatment [119]. In the group receiving FMT via colonoscopy, two patients reported prolonged satisfactory response up to 2 years after treatment [119].
Currently, seven clinical trials, mostly RCT studies, evaluating the effectiveness of FMT in PD are ongoing or have been completed. An overview of these studies is given in Table 3 [120–122]. Results from two studies are already available. The first study was an open-label study conducted on six patients with FMT administered via colonoscopy, showing positive effects on motor, non-motor and constipation symptoms in five out of six patients after 4 weeks [121]. The second RCT study evaluated 12 patients with constipation receiving oral multi-dose FMT for 12 weeks. After 9 months from treatment, FMT improved constipation and gut motility, and increased β-diversity and bacteria belonging to families Lactobacillaceae and Peptostreptococcaceae [120], which are known to protect the gut wall [123]. Overall, FMT was generally well tolerated and adverse events were mostly transient and self-limiting [118–121]. Recently, the protocol for a single-centre, prospective, self-controlled, interventional, safety and feasibility donor-FMT pilot study with randomisation and double-blinded allocation of donor faeces has been published by the FMT4PD study group in the Netherlands [122]. Sixteen PD patients with motor complications will receive FMT into the duodenum through a gastroscope and be followed up over 12 months. Primary outcomes will be feasibility and safety of FMT, while secondary outcomes will include changes in motor and non-motor symptoms, as well as alterations in GM composition [122].
Table 3.
Current clinical trials evaluating FMT in Parkinson disease patients
| Clinical trial ID | Location | Study design | No. of participants (enrolled or expected if study not completed) | Methods of transplantation | Primary outcomes | Recruitment status (reference if available) |
|---|---|---|---|---|---|---|
| NL9438 | Netherlands | RCT, double-blinded | 16 | Duodenal administration through gastroscopy | Safety and feasibility | Active [122] |
| NCT04837313 | China | Open label | 30 | NA | Changes in constipation (measured by Gastrointestinal Symptom Rating Scale and Wexner Constipation Scoring system) at 6 months | Active |
| NCT05204641 | Poland | RCT, double-blinded | 60 | Colonoscopy | Changes in MDS-UPDRS part III in OFF state at 12 months | Active, not recruiting |
| NCT03808389 | Ghent | RCT, quadruple-blinded | 49 | Nasojejunal administration | Changes in MDS-UPDRS and NMSS score at 3, 6, 12 months | Completed |
| NCT04854291 | Helsinki | RCT, quadruple-blinded | 51 | Intracaecal infusion | Changes in sum of MDS-UPDRS I-III at 6 months | Completed |
| NCT03876327 | Israel | Open label | 6 | Colonoscopy | Changes in MDS-UPDRS part III and constipation at 6 months | Completed [121] |
| NCT03671785 | United States | RCT, single-blinded (participant) | 12 | Lyophilized and encapsulated in enteric-coated capsules | GM diversity and richness at weeks 6 and 13, and months 4, 6 and 9 | Completed [120] |
FMT faecal microbiota transplantation, GM gut microbiota, MDS-UPDRS Movement Disorders Society Unified Parkinson’s Disease Rating Scale, NMSS non-motor symptoms scale for Parkinson’s disease, RCT randomised clinical trial
GM-Based Interventions: Potential Use as Disease-Modifying and Preventive Treatments
To date, studies testing GM-based interventions have been conducted in groups of patients with manifest PD, with the main goal to treat symptoms. Emerging evidence suggests, though, that the same interventions (e.g., prebiotics, probiotics, FMT) might be suitable as disease-modifying and preventive therapies.
On the one hand, longitudinal studies suggest that GM composition might predict PD progression. Lower abundance of genera Bifidobacterium, Barnesiella or Roseburia, or predominance of the Firmicutes-dominated enterotype were associated with faster progression in PD [76, 124–126], whereas abundance of genus Prevotella was protective towards deterioration [125]. Lower abundance of Ruminococcaceae and Actinobacteria was also associated with faster deterioration in global cognitive function [126].
On the other hand, groups at risk of future PD development, such as iRBD subjects, display similar changes in GM observed in manifest PD patients. When compared to controls, both iRBD and early PD patients showed depletion in butyrate-producing bacteria (e.g., Roseburia), and increased abundance of proinflammatory Collinsella and mucin-degrading Akkermansia [127]. A progressive gradient in GM changes was also observed between healthy, constipated, prodromal PD (characterised by constipation, hyposmia and probable RBD) and PD individuals, showing a gradual decrease in total abundance of strict anaerobes with anti-inflammatory properties (including Roseburia inulinivorans, Roseburia intestinalis and Faecalibacterium prausnitzii) [128]. These studies complete the existing in vivo and in vitro studies supporting a causative role of GM in PD presented in the Introduction [19–22].
Conclusions
Pharmacomicrobiomics is an emerging discipline that investigates how medications interact with gut bacteria [23]. Being involved in medication biotransformation, the GM can modify the bioavailability of medications [28], including levodopa. By improving levodopa absorption, the modulation of the GM potentially offers a wide range of benefits in PD, from optimal medication management to treatment of disabling motor symptoms secondary to peripheral levodopa resistance. In this scenario, the development of levodopa formulations combined with inhibitors of both host AADC and gut microbial TDC represent one of the most exciting areas of future research.
Next to pharmacological interventions, dietary interventions and use of pre/probiotics intended to change the GM composition represent a future avenue in the PD symptomatic therapeutic pipeline. Moreover, GM-focused interventions, in particular FMT, are now under evaluation as disease-modifying therapies in PD. The research in this field is at an early stage but must face many important challenges. How can we distinguish ‘responders’ and ‘non-responders’ to GM-oriented interventions? Which clinical measure outcomes, microbial features, or combination thereof, can define the success of an intervention? Would acute/short-term interventions have long-term effects? Beyond individual personal preferences and intervention adherence that might influence the outcome, would these interventions benefit all patients, or only certain subtypes of PD? Addressing these and several other challenges is of paramount importance for the success of GM-based interventions in PD.
Declarations
Funding
This research was funded in part by Aligning Science Across Parkinson’s (Grant number: ASAP-000420) through the Michael J. Fox Foundation for Parkinson’s Research (MJFF) and by the EU Joint Programme–Neurodegenerative Research (JPND) through the MRC grant code MR/T046007/1.
Conflicts of Interest
EM has received honoraria for a sponsored symposium from OMNIX PHARMA S.L. AHVS does not have any conflicts of interest to declare.
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
EM conceived the manuscript and wrote the first draft. AHVS conceived and reviewed the manuscript. All authors read and approved the final version of the manuscript, and agreed to be accountable for the work.
References
- 1.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 2.Savica R, Carlin JM, Grossardt BR, Bower JH, Ahlskog JE, Maraganore DM, et al. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology. 2009;73(21):1752–1758. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 4.Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta Neuropathol. 2014;127(2):235–241. doi: 10.1007/s00401-013-1214-6. [DOI] [PubMed] [Google Scholar]
- 5.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 6.Sprenger FS, Stefanova N, Gelpi E, Seppi K, Navarro-Otano J, Offner F, et al. Enteric nervous system alpha-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85(20):1761–1768. doi: 10.1212/WNL.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann Neurol. 2016;79(6):940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 8.Horsager J, Andersen KB, Knudsen K, Skjaerbaek C, Fedorova TD, Okkels N, et al. Brain-first versus body-first Parkinson's disease: a multimodal imaging case-control study. Brain. 2020;143(10):3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. 2017;88(21):1996–2002. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sorensen HT. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson's Disease. Neuron. 2019;103(4):627–41e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 13.Aho VTE, Houser MC, Pereira PAB, Chang J, Rudi K, Paulin L, et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol Neurodegener. 2021;16(1):6. doi: 10.1186/s13024-021-00427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS ONE. 2011;6(12):e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulak A, Koszewicz M, Panek-Jeziorna M, Koziorowska-Gawron E, Budrewicz S. Fecal calprotectin as a marker of the gut immune system activation is elevated in Parkinson's disease. Front Neurosci. 2019;13:992. doi: 10.3389/fnins.2019.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, et al. Role of TLR4 in the gut-brain axis in Parkinson's disease: a translational study from men to mice. Gut. 2019;68(5):829–843. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 17.Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Burmann J, Fassbender K, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson's disease. Parkinsonism Relat Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson's disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi: 10.1038/s41531-021-00156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matheoud D, Cannon T, Voisin A, Penttinen AM, Ramet L, Fahmy AM, et al. Intestinal infection triggers Parkinson's disease-like symptoms in Pink1(-/-) mice. Nature. 2019;571(7766):565–569. doi: 10.1038/s41586-019-1405-y. [DOI] [PubMed] [Google Scholar]
- 20.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(6):1469–80e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Hurley MJ, Menozzi E, Koletsi S, Bates R, Gegg ME, Chau KY, et al. alpha-Synuclein expression in response to bacterial ligands and metabolites in gut enteroendocrine cells: an in vitro proof of concept study. Brain Commun. 2023;5(6):fcad285. doi: 10.1093/braincomms/fcad285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad R, Rizkallah MR, Aziz RK. Gut Pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012;4(1):16. doi: 10.1186/1757-4749-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foltynie T, Bruno V, Fox S, Kuhn AA, Lindop F, Lees AJ. Medical, surgical, and physical treatments for Parkinson's disease. Lancet. 2024;403(10423):305–324. doi: 10.1016/S0140-6736(23)01429-0. [DOI] [PubMed] [Google Scholar]
- 25.Leta V, Klingelhoefer L, Longardner K, Campagnolo M, Levent HC, Aureli F, et al. Gastrointestinal barriers to levodopa transport and absorption in Parkinson's disease. Eur J Neurol. 2023;30(5):1465–1480. doi: 10.1111/ene.15734. [DOI] [PubMed] [Google Scholar]
- 26.Beckers M, Bloem BR, Verbeek MM. Mechanisms of peripheral levodopa resistance in Parkinson's disease. NPJ Parkinsons Dis. 2022;8(1):56. doi: 10.1038/s41531-022-00321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hey G, Nair N, Klann E, Gurrala A, Safarpour D, Mai V, et al. Therapies for Parkinson's disease and the gut microbiome: evidence for bidirectional connection. Front Aging Neurosci. 2023;15:1151850. doi: 10.3389/fnagi.2023.1151850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Han Y, Huang W, Jin M, Gao Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm Sin B. 2021;11(7):1789–1812. doi: 10.1016/j.apsb.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kessel SP, de Jong HR, Winkel SL, van Leeuwen SS, Nelemans SA, Permentier H, et al. Gut bacterial deamination of residual levodopa medication for Parkinson's disease. BMC Biol. 2020;18(1):137. doi: 10.1186/s12915-020-00876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Kessel SP, El Aidy S. Contributions of Gut bacteria and diet to drug pharmacokinetics in the treatment of Parkinson's disease. Front Neurol. 2019;10:1087. doi: 10.3389/fneur.2019.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camargo SM, Vuille-dit-Bille RN, Mariotta L, Ramadan T, Huggel K, Singer D, et al. The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson's disease. J Pharmacol Exp Ther. 2014;351(1):114–123. doi: 10.1124/jpet.114.216317. [DOI] [PubMed] [Google Scholar]
- 32.Rusch C, Flanagan R, Suh H, Subramanian I. To restrict or not to restrict? Practical considerations for optimizing dietary protein interactions on levodopa absorption in Parkinson's disease. NPJ Parkinsons Dis. 2023;9(1):98. doi: 10.1038/s41531-023-00541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barichella M, Marczewska A, De Notaris R, Vairo A, Baldo C, Mauri A, et al. Special low-protein foods ameliorate postprandial off in patients with advanced Parkinson's disease. Mov Disord. 2006;21(10):1682–1687. doi: 10.1002/mds.21003. [DOI] [PubMed] [Google Scholar]
- 34.Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975;27(2):135–206. [PubMed] [Google Scholar]
- 35.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445):eaau6323. 10.1126/science.aau6323. PMID: 31196984; PMCID: PMC7745125. [DOI] [PMC free article] [PubMed]
- 36.Sandler M, Goodwin BL, Ruthven CR. Therapeutic implications in Parkinsonism of m-tyramine formation from L-dopa in man. Nature. 1971;229(5284):414–415. doi: 10.1038/229414a0. [DOI] [PubMed] [Google Scholar]
- 37.Goldin BR, Peppercorn MA, Goldman P. Contributions of host and intestinal microflora in the metabolism of L-dopa by the rat. J Pharmacol Exp Ther. 1973;186(1):160–166. [PubMed] [Google Scholar]
- 38.van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G, El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson's disease. Nat Commun. 2019;10(1):310. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, He X, Mo C, Liu X, Li J, Yan Z, et al. Association between microbial tyrosine decarboxylase gene and levodopa responsiveness in patients with Parkinson disease. Neurology. 2022;99(22):e2443–e2453. doi: 10.1212/WNL.0000000000201204. [DOI] [PubMed] [Google Scholar]
- 40.Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013;341(6143):295–298. doi: 10.1126/science.1235872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25(3):237–240. doi: 10.1159/000103892. [DOI] [PubMed] [Google Scholar]
- 42.Losurdo G, Salvatore D'Abramo F, Indellicati G, Lillo C, Ierardi E, Di Leo A. The influence of small intestinal bacterial overgrowth in digestive and extra-intestinal disorders. Int J Mol Sci. 2020;21(10). [DOI] [PMC free article] [PubMed]
- 43.Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, et al. The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord. 2013;28(9):1241–1249. doi: 10.1002/mds.25522. [DOI] [PubMed] [Google Scholar]
- 44.Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM, et al. Small intestinal bacterial overgrowth in Parkinson's disease. Parkinsonism Relat Disord. 2014;20(5):535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Gabrielli M, Bonazzi P, Scarpellini E, Bendia E, Lauritano EC, Fasano A, et al. Prevalence of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord. 2011;26(5):889–892. doi: 10.1002/mds.23566. [DOI] [PubMed] [Google Scholar]
- 46.Vizcarra JA, Wilson-Perez HE, Fasano A, Espay AJ. Small intestinal bacterial overgrowth in Parkinson's disease: tribulations of a trial. Parkinsonism Relat Disord. 2018;54:110–112. doi: 10.1016/j.parkreldis.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Shen X, Yang H, Wu Y, Zhang D, Jiang H. Meta-analysis: Association of Helicobacter pylori infection with Parkinson's diseases. Helicobacter. 2017;22(5). [DOI] [PubMed]
- 48.Huang HK, Wang JH, Lei WY, Chen CL, Chang CY, Liou LS. Helicobacter pylori infection is associated with an increased risk of Parkinson's disease: a population-based retrospective cohort study. Parkinsonism Relat Disord. 2018;47:26–31. doi: 10.1016/j.parkreldis.2017.11.331. [DOI] [PubMed] [Google Scholar]
- 49.Tan AH, Mahadeva S, Marras C, Thalha AM, Kiew CK, Yeat CM, et al. Helicobacter pylori infection is associated with worse severity of Parkinson's disease. Parkinsonism Relat Disord. 2015;21(3):221–225. doi: 10.1016/j.parkreldis.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Pierantozzi M, Pietroiusti A, Galante A, Sancesario G, Lunardi G, Fedele E, et al. Helicobacter pylori-induced reduction of acute levodopa absorption in Parkinson's disease patients. Ann Neurol. 2001;50(5):686–687. doi: 10.1002/ana.1267. [DOI] [PubMed] [Google Scholar]
- 51.Pierantozzi M, Pietroiusti A, Sancesario G, Lunardi G, Fedele E, Giacomini P, et al. Reduced L-dopa absorption and increased clinical fluctuations in Helicobacter pylori-infected Parkinson's disease patients. Neurol Sci. 2001;22(1):89–91. doi: 10.1007/s100720170061. [DOI] [PubMed] [Google Scholar]
- 52.Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66(12):1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]
- 53.Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL. Helicobacter pylori infection and motor fluctuations in patients with Parkinson's disease. Mov Disord. 2008;23(12):1696–1700. doi: 10.1002/mds.22190. [DOI] [PubMed] [Google Scholar]
- 54.Mridula KR, Borgohain R, Chandrasekhar Reddy V, Bandaru V, Suryaprabha T. Association of Helicobacter pylori with Parkinson's Disease. J Clin Neurol. 2017;13(2):181–186. doi: 10.3988/jcn.2017.13.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan AH, Lim SY, Mahadeva S, Loke MF, Tan JY, Ang BH, et al. Helicobacter pylori eradication in Parkinson's disease: a randomized placebo-controlled trial. Mov Disord. 2020;35(12):2250–2260. doi: 10.1002/mds.28248. [DOI] [PubMed] [Google Scholar]
- 56.Narozanska E, Bialecka M, Adamiak-Giera U, Gawronska-Szklarz B, Soltan W, Schinwelski M, et al. Pharmacokinetics of levodopa in patients with Parkinson disease and motor fluctuations depending on the presence of Helicobacter pylori infection. Clin Neuropharmacol. 2014;37(4):96–99. doi: 10.1097/WNF.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 57.Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y). 2007;3(2):112–122. [PMC free article] [PubMed] [Google Scholar]
- 58.Niehues M, Hensel A. In-vitro interaction of L-dopa with bacterial adhesins of Helicobacter pylori: an explanation for clinicial differences in bioavailability? J Pharm Pharmacol. 2009;61(10):1303–1307. doi: 10.1211/jpp/61.10.0005. [DOI] [PubMed] [Google Scholar]
- 59.Baizabal-Carvallo JF, Alonso-Juarez M, Fekete R. Intestinal decontamination therapy for dyskinesia and motor fluctuations in Parkinson's disease. Front Neurol. 2021;12:729961. doi: 10.3389/fneur.2021.729961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palacios N, Hannoun A, Flahive J, Ward D, Goostrey K, Deb A, Smith KM. Effect of levodopa initiation on the gut microbiota in Parkinson's disease. Front Neurol. 2021;12:574529. doi: 10.3389/fneur.2021.574529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heintz-Buschart A, Pandey U, Wicke T, Sixel-Doring F, Janzen A, Sittig-Wiegand E, et al. The nasal and gut microbiome in Parkinson's disease and idiopathic rapid eye movement sleep behavior disorder. Mo Disord. 2018;33(1):88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weis S, Schwiertz A, Unger MM, Becker A, Fassbender K, Ratering S, et al. Effect of Parkinson's disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinsons Dis. 2019;5:28. doi: 10.1038/s41531-019-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melis M, Vascellari S, Santoru ML, Oppo V, Fabbri M, Sarchioto M, et al. Gut microbiota and metabolome distinctive features in Parkinson disease: focus on levodopa and levodopa-carbidopa intrajejunal gel. Eur J Neurol. 2021;28(4):1198–1209. doi: 10.1111/ene.14644. [DOI] [PubMed] [Google Scholar]
- 64.Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149. doi: 10.1016/S1474-4422(13)70293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lubomski M, Xu X, Holmes AJ, Yang JYH, Sue CM, Davis RL. The impact of device-assisted therapies on the gut microbiome in Parkinson's disease. J Neurol. 2022;269(2):780–795. doi: 10.1007/s00415-021-10657-9. [DOI] [PubMed] [Google Scholar]
- 66.Benjamin MM, Datta AR. Acid tolerance of enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1995;61(4):1669–1672. doi: 10.1128/aem.61.4.1669-1672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamanishi Y, Choudhury ME, Yoshida A, Hosokawa Y, Miyaue N, Tada S, et al. Impact of Intestinal Bacteria on Levodopa Pharmacokinetics in LCIG Therapy. Mov Disord Clin Pract. 2022;9(3):362–368. doi: 10.1002/mdc3.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, et al. Unraveling gut microbiota in Parkinson's disease and atypical parkinsonism. Mov Disord. 2019;34(3):396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 69.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 70.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, et al. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grun D, Zimmer VC, Kauffmann J, Spiegel J, Dillmann U, Schwiertz A, et al. Impact of oral COMT-inhibitors on gut microbiota and short chain fatty acids in Parkinson's disease. Parkinsonism Relat Disord. 2020;70:20–22. doi: 10.1016/j.parkreldis.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Fu SC, Lee CH, Hsieh YC, Wu PH, Lin SH, Wang H. A pilot study exploring the association of entacapone, gut microbiota, and the subsequent side effects in patients with Parkinson's disease. Front Cell Infect Microbiol. 2022;12:837019. doi: 10.3389/fcimb.2022.837019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Kessel SP, Bullock A, van Dijk G, El Aidy S. Parkinson's disease medication alters small intestinal motility and microbiota composition in healthy rats. mSystems. 2022;7(1):e0119121. doi: 10.1128/msystems.01191-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallen ZD, Appah M, Dean MN, Sesler CL, Factor SA, Molho E, et al. Characterizing dysbiosis of gut microbiome in PD: evidence for overabundance of opportunistic pathogens. NPJ Parkinsons Dis. 2020;6:11. doi: 10.1038/s41531-020-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Kessel SP, Auvinen P, Scheperjans F, El Aidy S. Gut bacterial tyrosine decarboxylase associates with clinical variables in a longitudinal cohort study of Parkinsons disease. NPJ Parkinsons Dis. 2021;7(1):115. doi: 10.1038/s41531-021-00260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lubomski M, Xu X, Holmes AJ, Muller S, Yang JYH, Davis RL, Sue CM. The gut microbiome in Parkinson's disease: a longitudinal study of the impacts on disease progression and the use of device-assisted therapies. Front Aging Neurosci. 2022;14:875261. doi: 10.3389/fnagi.2022.875261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varesi A, Campagnoli LIM, Fahmideh F, Pierella E, Romeo M, Ricevuti G, et al. The interplay between gut microbiota and Parkinson's disease: implications on diagnosis and treatment. Int J Mol Sci. 2022;23(20). [DOI] [PMC free article] [PubMed]
- 78.Barichella M, Cereda E, Pinelli G, Iorio L, Caroli D, Masiero I, et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology. 2019;93(5):e485–e496. doi: 10.1212/WNL.0000000000007858. [DOI] [PubMed] [Google Scholar]
- 79.Coe S, Andreoli D, George M, Collett J, Reed A, Cossington J, et al. A feasibility study to determine whether the daily consumption of flavonoid-rich pure cocoa has the potential to reduce fatigue and fatigability in people with Parkinson's (pwP) Clin Nutr ESPEN. 2022;48:68–73. doi: 10.1016/j.clnesp.2022.01.023. [DOI] [PubMed] [Google Scholar]
- 80.Cucca A, Mazzucco S, Bursomanno A, Antonutti L, Di Girolamo FG, Pizzolato G, et al. Amino acid supplementation in l-dopa treated Parkinson's disease patients. Clin Nutr. 2015;34(6):1189–1194. doi: 10.1016/j.clnu.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 81.da Silva TM, Munhoz RP, Alvarez C, Naliwaiko K, Kiss A, Andreatini R, Ferraz AC. Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J Affect Disord. 2008;111(2–3):351–359. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 82.DiFrancisco-Donoghue J, Lamberg EM, Rabin E, Elokda A, Fazzini E, Werner WG. Effects of exercise and B vitamins on homocysteine and glutathione in Parkinson's disease: a randomized trial. Neurodegener Dis. 2012;10(1–4):127–134. doi: 10.1159/000333790. [DOI] [PubMed] [Google Scholar]
- 83.Paknahad Z, Sheklabadi E, Derakhshan Y, Bagherniya M, Chitsaz A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson's disease: a randomized clinical controlled trial. Complement Ther Med. 2020;50:102366. doi: 10.1016/j.ctim.2020.102366. [DOI] [PubMed] [Google Scholar]
- 84.Paknahad Z, Sheklabadi E, Moravejolahkami AR, Chitsaz A, Hassanzadeh A. The effects of Mediterranean diet on severity of disease and serum Total Antioxidant Capacity (TAC) in patients with Parkinson's disease: a single center, randomized controlled trial. Nutr Neurosci. 2022;25(2):313–320. doi: 10.1080/1028415X.2020.1751509. [DOI] [PubMed] [Google Scholar]
- 85.Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: A pilot randomized controlled trial. Mov Disord. 2018;33(8):1306–1314. doi: 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rusch C, Beke M, Tucciarone L, Dixon K, Nieves C, Jr, Mai V, et al. Effect of a Mediterranean diet intervention on gastrointestinal function in Parkinson's disease (the MEDI-PD study): study protocol for a randomised controlled trial. BMJ Open. 2021;11(9):e053336. doi: 10.1136/bmjopen-2021-053336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Improved nutritional status is related to improved quality of life in Parkinson's disease. BMC Neurol. 2014;18(14):212. doi: 10.1186/s12883-014-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taghizadeh M, Tamtaji OR, Dadgostar E, Daneshvar Kakhaki R, Bahmani F, Abolhassani J, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Neurochem Int. 2017;108:183–189. doi: 10.1016/j.neuint.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 89.Tosukhowong P, Boonla C, Dissayabutra T, Kaewwilai L, Muensri S, Chotipanich C, et al. Biochemical and clinical effects of Whey protein supplementation in Parkinson's disease: a pilot study. J Neurol Sci. 2016;15(367):162–170. doi: 10.1016/j.jns.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 90.Solch RJ, Aigbogun JO, Voyiadjis AG, Talkington GM, Darensbourg RM, O'Connell S, et al. Mediterranean diet adherence, gut microbiota, and Alzheimer's or Parkinson's disease risk: a systematic review. J Neurol Sci. 2022;15(434):120166. doi: 10.1016/j.jns.2022.120166. [DOI] [PubMed] [Google Scholar]
- 91.Rusch C, Beke M, Tucciarone L, Nieves C, Jr, Ukhanova M, Tagliamonte MS, et al. Mediterranean diet adherence in people with Parkinson's disease reduces constipation symptoms and changes fecal microbiota after a 5-week single-arm pilot study. Front Neurol. 2021;12:794640. doi: 10.3389/fneur.2021.794640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pietrzak D, Kasperek K, Rekawek P, Piatkowska-Chmiel I. The therapeutic role of ketogenic diet in neurological disorders. Nutrients. 2022;14(9). [DOI] [PMC free article] [PubMed]
- 93.Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64(4):728–730. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- 94.Hegelmaier T, Lebbing M, Duscha A, Tomaske L, Tonges L, Holm JB, et al. Interventional influence of the intestinal microbiome through dietary intervention and bowel cleansing might improve motor symptoms in Parkinson's disease. Cells. 2020;9(2). [DOI] [PMC free article] [PubMed]
- 95.Mirmiran P, Bahadoran Z, Gaeini Z. Common limitations and challenges of dietary clinical trials for translation into clinical practices. Int J Endocrinol Metab. 2021;19(3):e108170. doi: 10.5812/ijem.108170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 97.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics—a review. J Food Sci Technol. 2015;52(12):7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cantu-Jungles TM, Rasmussen HE, Hamaker BR. Potential of prebiotic butyrogenic fibers in Parkinson's disease. Front Neurol. 2019;10:663. doi: 10.3389/fneur.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hall DA, Voigt RM, Cantu-Jungles TM, Hamaker B, Engen PA, Shaikh M, et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson's disease participants. Nat Commun. 2023;14(1):926. doi: 10.1038/s41467-023-36497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Astarloa R, Mena MA, Sanchez V, de la Vega L, de Yebenes JG. Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin Neuropharmacol. 1992;15(5):375–380. doi: 10.1097/00002826-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 102.Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EM. Constipation in Parkinson's disease: objective assessment and response to psyllium. Mov Disord. 1997;12(6):946–951. doi: 10.1002/mds.870120617. [DOI] [PubMed] [Google Scholar]
- 103.Becker A, Schmartz GP, Groger L, Grammes N, Galata V, Philippeit H, et al. Effects of resistant starch on symptoms, fecal markers, and gut microbiota in Parkinson's disease—the RESISTA-PD Trial. Genom ProteomBioinform. 2022;20(2):274–287. doi: 10.1016/j.gpb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 105.Cassani E, Privitera G, Pezzoli G, Pusani C, Madio C, Iorio L, Barichella M. Use of probiotics for the treatment of constipation in Parkinson's disease patients. Minerva Gastroenterol Dietol. 2011;57(2):117–121. [PubMed] [Google Scholar]
- 106.Barichella M, Pacchetti C, Bolliri C, Cassani E, Iorio L, Pusani C, et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: an RCT. Neurology. 2016;87(12):1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 107.Du Y, Li Y, Xu X, Li R, Zhang M, Cui Y, et al. Probiotics for constipation and gut microbiota in Parkinson's disease. Parkinsonism Relat Disord. 2022;103:92–97. doi: 10.1016/j.parkreldis.2022.08.022. [DOI] [PubMed] [Google Scholar]
- 108.Georgescu D, Ancusa OE, Georgescu LA, Ionita I, Reisz D. Nonmotor gastrointestinal disorders in older patients with Parkinson's disease: is there hope? Clin Interv Aging. 2016;11:1601–1608. doi: 10.2147/CIA.S106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ibrahim A, Ali RAR, Manaf MRA, Ahmad N, Tajurruddin FW, Qin WZ, et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson's disease: a randomised controlled trial. PLoS ONE. 2020;15(12):e0244680. doi: 10.1371/journal.pone.0244680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun H, Zhao F, Liu Y, Ma T, Jin H, Quan K, et al. Probiotics synergized with conventional regimen in managing Parkinson's disease. NPJ Parkinsons Dis. 2022;8(1):62. doi: 10.1038/s41531-022-00327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, et al. Clinical and metabolic response to probiotic administration in people with Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(3):1031–1035. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 112.Tan AH, Lim SY, Chong KK, Manap MA, Hor JW, Lim JL, et al. Probiotics for constipation in Parkinson disease: a randomized placebo-controlled study. Neurology. 2021;96(5):e772–e782. doi: 10.1212/WNL.0000000000010998. [DOI] [PubMed] [Google Scholar]
- 113.Yang X, He X, Xu S, Zhang Y, Mo C, Lai Y, et al. Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson's disease. Food Funct. 2023;14(15):6828–6839. doi: 10.1039/D3FO00728F. [DOI] [PubMed] [Google Scholar]
- 114.Borzabadi S, Oryan S, Eidi A, Aghadavod E, Daneshvar Kakhaki R, Tamtaji OR, et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson's disease: a randomized, double-blind placebocontrolled, trial. Arch Iran Med. 2018;21(7):289–295. [PubMed] [Google Scholar]
- 115.Seppi K, Ray Chaudhuri K, Coelho M, Fox SH, Katzenschlager R, Perez Lloret S, et al. Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov Disord. 2019;34(2):180–198. doi: 10.1002/mds.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G, Zhang D. Fecal microbiota transplantation protects rotenone-induced Parkinson's disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome. 2021;9(1):226. doi: 10.1186/s40168-021-01107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Baunwall SMD, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, Hvas CL. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;29–30:100642. doi: 10.1016/j.eclinm.2020.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuai XY, Yao XH, Xu LJ, Zhou YQ, Zhang LP, Liu Y, et al. Evaluation of fecal microbiota transplantation in Parkinson's disease patients with constipation. Microb Cell Fact. 2021;20(1):98. doi: 10.1186/s12934-021-01589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xue LJ, Yang XZ, Tong Q, Shen P, Ma SJ, Wu SN, et al. Fecal microbiota transplantation therapy for Parkinson's disease: a preliminary study. Medicine (Baltimore) 2020;99(35):e22035. doi: 10.1097/MD.0000000000022035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DuPont HL, Suescun J, Jiang ZD, Brown EL, Essigmann HT, Alexander AS, et al. Fecal microbiota transplantation in Parkinson's disease—a randomized repeat-dose, placebo-controlled clinical pilot study. Front Neurol. 2023;14:1104759. doi: 10.3389/fneur.2023.1104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Segal A, Zlotnik Y, Moyal-Atias K, Abuhasira R, Ifergane G. Fecal microbiota transplant as a potential treatment for Parkinson's disease—a case series. Clin Neurol Neurosurg. 2021;207:106791. doi: 10.1016/j.clineuro.2021.106791. [DOI] [PubMed] [Google Scholar]
- 122.Vendrik KE, Chernova VO, Kuijper EJ, Terveer EM, van Hilten JJ, Contarino MF. Safety and feasibility of faecal microbiota transplantation for patients with Parkinson's disease: a protocol for a self-controlled interventional donor-FMT pilot study. BMJ Open. 2023;13(10):e071766. doi: 10.1136/bmjopen-2023-071766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T. Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol. 2016;69(3):187–203. doi: 10.1136/jclinpath-2015-202976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, Hirayama M. Progression of Parkinson's disease is associated with gut dysbiosis: two-year follow-up study. PLoS ONE. 2017;12(11):e0187307. doi: 10.1371/journal.pone.0187307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aho VTE, Pereira PAB, Voutilainen S, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Gut microbiota in Parkinson's disease: temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cilia R, Piatti M, Cereda E, Bolliri C, Caronni S, Ferri V, et al. Does Gut microbiota influence the course of Parkinson's disease? A 3-year prospective exploratory study in de novo patients. J Parkinsons Dis. 2021;11(1):159–170. doi: 10.3233/JPD-202297. [DOI] [PubMed] [Google Scholar]
- 127.Huang B, Chau SWH, Liu Y, Chan JWY, Wang J, Ma SL, et al. Gut microbiome dysbiosis across early Parkinson's disease, REM sleep behavior disorder and their first-degree relatives. Nat Commun. 2023;14(1):2501. doi: 10.1038/s41467-023-38248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Palacios N, Wilkinson J, Bjornevik K, Schwarzschild MA, McIver L, Ascherio A, Huttenhower C. Metagenomics of the gut microbiome in Parkinson's disease: prodromal changes. Ann Neurol. 2023;94(3):486–501. doi: 10.1002/ana.26719. [DOI] [PMC free article] [PubMed] [Google Scholar]


