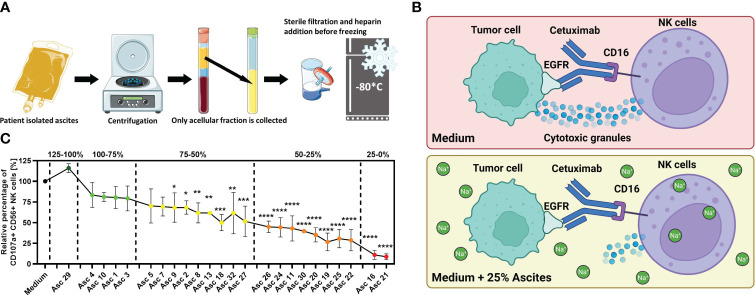
Figure 1.
Malignant peritoneal ascites impairs NK cell effector functions in vitro. (A) Processing of ascites samples. Graphical illustration of initial processing of ascites samples derived from patients with malignant ascites and storage of ascitic fluid after depletion of cellular components. (B) Illustration of the NK–tumor cell coculture system under the ADCC condition. This experimental setup was used to assess the effects of ascites or healthy donor serum on NK cell effector function. The same setup in the previous study was used, which revealed that malignant ascites is a sodium-imbalanced environment (24). Illustrations were created with BioRender and Sevier Medical Art. (C) Antibody-dependent cellular cytotoxicity (ADCC) of NK cells (NK) in the presence of selected ascites samples. Resting healthy donor NK cells were coincubated in 1:1 ratio with IGROV1- cells for 6 h with the addition of ADCC-inducing anti-EGFR-antibody Cetuximab (1 µg/mL) and benign ascites (Asc 29) or malignant ascites (all other 23 samples). We could differentiate samples with only weak (green), with intermediate (yellow), strong (orange), and very strong (red) inhibitory potential and sample no. 29 with no inhibition of NK ADCC (dark green) (24). Each datapoint represents one healthy NK cell donor. The relative percentages are shown after normalization to normal medium control. Data are presented as individual values with mean value as center of error bar ± standard deviation. Each datapoint represents one healthy donor. The normalization was done according to normal medium control. For significance testing, ordinary one-way (C) ANOVA and Dunnett’s post-hoc test were used.