Summary
The determination of an individual’s geographic origin is an essential aspect of forensic investigations. When primary identifiers cannot be used to make a positive identification, isotope analysis can be utilized to provide new leads. Modern reference data are essential for accurate interpretation of human isotopic data in terms of diet and origin. This article presents Sr-O-C-N-H isotope data of modern individuals (hair, dental enamel, and dentine collagen) and drinking water from the Netherlands. The δ15N values of human hair fall within the range of values observed worldwide and cannot be utilized to differentiate from other countries. Distinct disparities in the hair δ13C are evident between European countries and other regions, making it possible to exclude the Netherlands as a region of origin. Comparing Dutch dental isotope data to those of other nations has proven difficult due to the limited availability of reference data. The same limitation applies to tap water δ2H data.
Subject areas: structures
Graphical abstract
Highlights
-
•
Sr-O-C-N-H isotope data of modern humans and drinking water from the Netherlands
-
•
δ15N values of human hair cannot be utilized to differentiate from other countries
-
•
Variations in hair δ13C enable the exclusion the Netherlands as a region of origin
-
•
Limited availability of reference data makes interpretation of data difficult
structures
Introduction
Identification of human remains is paramount in forensic investigations. The primary identifiers used for identification are DNA profiling, fingerprint ridge analysis, roentgenography, and comparative analysis of dental records.1,2 However, these techniques require a known record from the person of interest, or family in the case of DNA, to make a positive identification. In cases where primary identifiers cannot be used or provide no match, other techniques must be utilized to provide new leads that could reveal the identity of the deceased individuals. Isotope analysis has become more prominent in forensic science, since first being used to aid human identification in 1982, where the composition of stable isotopes in human tissue was seen to vary from person to person.3,4,5,6,7,8,9,10,11,12 Nevertheless, its full potential has not been exploited, and several isotope systems are yet to be applied in medicolegal casework.13,14 Most applications of forensic isotope analysis focus on the reconstructions of the timeline of geographic movements and dietary habits.6,15,16 The isotope systems of, inter alia, strontium (87Sr/86Sr), lead (20xPb/20xPb), oxygen (δ18O), and hydrogen (δ2H) can be used to gain valuable insights into the geological or even geographical location the person grew up in or where the deceased has spent the last years to months of life.17,18,19,20 Carbon (δ13C), nitrogen (δ15N), sulfur (δ34S), but also, less commonly used in current practice, calcium (δ44/42Ca), zinc (δ66Zn), and stable strontium (δ88Sr) can provide valuable data on an individual’s diet.21,22,23
The elements mentioned previously are incorporated into our tissues through the food and water we consume and the air we breathe, as well as other factors, such as bathing water.24,25,26,27,28,29,30,31 The isotope ratios measured in various tissues, such as hair keratin, bones (apatite and collagen), and dental elements, provide insights into provenance and diet during various stages of life. Dental enamel of permanent dentition, for instance, mineralizes during childhood, incorporating the isotope ratio of the foods consumed during that specific period.32 In contrast, due to bone remodeling, the isotope composition of bone apatite and collagen can vary in and between the skeletal elements. For example, depending on the age at death, the isotope composition of trabecular bone represents the average isotope composition of the consumed food during the approximately last five years of life, while cortical bone can represent the last decades prior to death.5,33,34,35,36,37,38 Isotopically speaking, the collagen will be representative of the dietary protein intake, with the bioapatite being representative of the whole diet.39,40,41 Theoretically, using multiple isotope systems and multiple types of human tissues, very specific insights can be gained about recent and childhood provenance and diet. However, the accuracy of the interpretation of isotope data heavily depends on the presence and quality of relevant reference datasets. Absence of reference data or the presence of small, unrepresentative datasets may often lead to the identification of large potential regions of origin, hampering the application potential of isotope data in medicolegal casework.
Isotope research in archaeology is based on the premise that the palaeodiet was dominated by locally grown foods. However, the isotopic disparities in the Dutch geological subsurface (Sr) and nutritional availability, which were discernible in prehistoric and historical eras through the composition of consumed foods and consequently in individuals, are not always observable in the contemporary period.18,42 Today, globalization of food production and distribution has led to some disconnection of consumers from the location of food production. This “global supermarket-diet” has the potential to override the “local” (i.e., geological and environmental) isotope signatures, leading to isotope ratios that are not indicative of specific geological or geographical origins. Consequently, available strontium isotope landscape maps, Sr isoscapes in short, that are frequently used for identification of potential regions of origin in archaeological isotope research, e.g., in the study by West et al., Kootker et al., Willmes et al., Snoeck et al., and Lugli et al.,43,44,45,46,47 are not necessarily applicable in forensic casework. Relevant reference databases and isoscape maps are specifically required for the use in modern (forensic) contexts.18,24,48,49,50
The application of isotope analysis in Dutch forensic casework increased significantly since its introduction in 2008. The collaboration between the Netherlands Forensic Institute (NFI), the national police force, and the Vrije Universiteit Amsterdam has resulted in a wider acceptance of the method to gather new information about the geographical origin and ultimately the identification of the unknown dead. In 2023, INTERPOL and the Belgian, Dutch, and German police launched “Operation Identify Me”, an ultimate attempt to identify 22 unknown females.51 The information in cases referring to possible regions of (childhood) origin is extracted from isotope analysis. As the significance of isotope analysis for forensic sciences is increasing, it is essential to increase the accuracy of the interpreted isotope data. Consequently, the growing and successful implementation of isotope research in Dutch forensic science calls for the need to expand the available relevant reference datasets and the development of reference maps specifically for modern (forensic) research.
Unfortunately, to date, reference or baseline isotope data from modern human individuals from the Netherlands are scarce. However, a recent paper reported 153 modern human enamel and 143 tap water 87Sr/86Sr measurements from the Netherlands, defining a first “Dutch” strontium isotope signature with Sr ratios ranging between 0.7088 and 0.7099.18 In contrast, there are extremely limited available modern Dutch human C-N-O-H isotope data.15,17 Therefore, this paper presents newly generated isotope data from tap water (O-H), dentine collagen and hair keratin (C-N), and dental enamel (Sr-C-O), updating (Sr) and expanding (O-C-N-H) the published isotopic reference datasets. This publication will contribute to a better understanding of the Sr-C-N-O-H isotope composition of or accessible to the modern Dutch population and add to the ever-growing modern-day human tissue and tap water database of the Netherlands, while tying the new data into the previously published data.
Results
The Dutch human hair, dental elements, and tap water database now consists of 776 data points for the isotope systems of carbon, nitrogen, oxygen, hydrogen, and strontium. A detailed breakdown of the data added by this study can be found in the STAR Methods section. All δ15N and δ13C isotope values for human hair; δ18O, δ13C values and 87Sr/86Sr for dental enamel; δ13C and δ15N isotope values for dentine-derived collagen, and δ18O, δ2H values and 87Sr/86Sr for tap water can be found in the Supplementary Data file. An overview of the descriptive statistics of the results can be found in Table 1.
Table 1.
Descriptive statistics of the results for hair keratin, dental enamel (bioapatite), dentine (collagen), and tap water samples
| Hair |
Enamel |
Dental collagen |
Tap water |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ15N | δ13C | δ18O | δ13C | 87Sr/86Sr | δ15N | δ13C | δ18O | δ2H | 87Sr/86Sr | |
| Sample size (n) | 28 | 28 | 73 | 73 | 159 | 38 | 38 | 98 | 98 | 143 |
| Minimum | 8.4 | −21.6 | −7.4 | −14.8 | 0.70896 | 10.2 | −22.0 | −9.5 | −66.9 | 0.70837 |
| Maximum | 10.6 | −20.4 | −5.0 | −12.8 | 0.70942 | 11.9 | −19.9 | −4.4 | −30.1 | 0.71278 |
| Mean | 9.2 | −20.9 | −6.5 | −13.9 | 0.70919 | 11.0 | −20.7 | −7.3 | −49.7 | 0.70927 |
| SD | 0.4 | 0.3 | 0.5 | 0.5 | 0.00024 | 0.4 | 0.4 | 0.9 | 6.5 | 0.00067 |
Key: δ15N, δ13C, and δ18O in per mil (‰) versus AIR, VPDB, and VPDB (enamel) and VSMOW (tap water), respectively. SD, standard deviation (σ).
Human hair
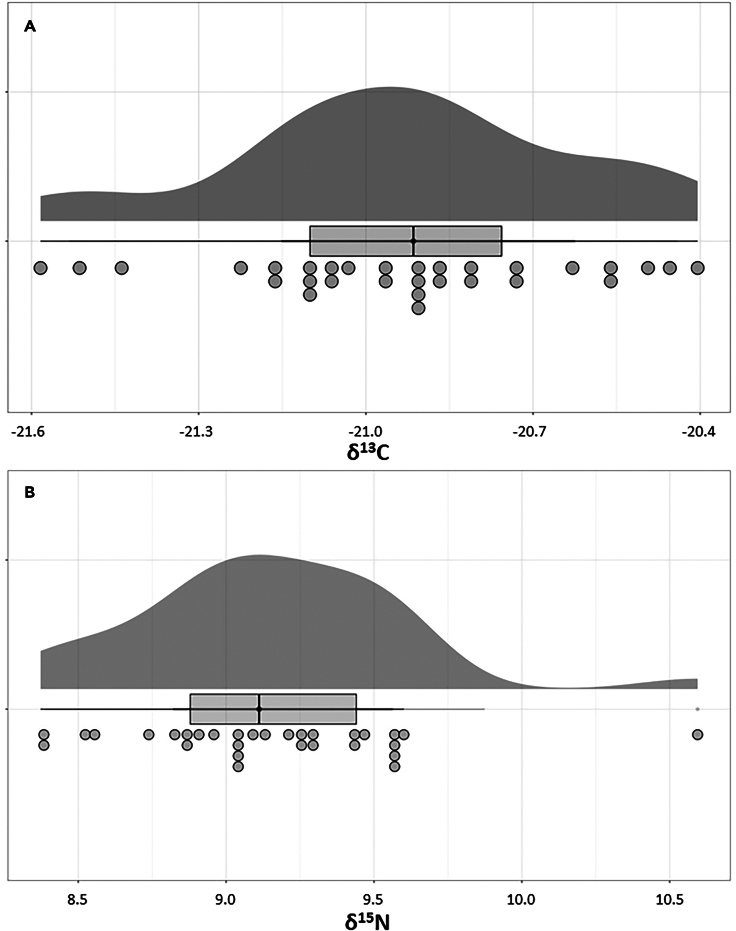
A total of 28 hair samples were analyzed for their δ15N and δ13C isotope values (Supplementary Data). The metadata from the questionnaires completed by the donors can also be found in the Supplementary Data. No formal analyses were conducted on the metadata; however, they can be used for future research. The δ15N values recorded range from 8.4‰ to 10.6‰, with a mean of 9.2‰ ± 0.4‰ (1σ). The δ13C isotope values average −20.9‰ ± 0.3‰ (1σ), with the minimum being −21.6‰ and the maximum being −20.4‰ (Table 1). A visual representation of the distribution of these isotope values can be found in Figure 1.
Figure 1.
Raincloud plots of the isotope values found in modern human hair from the Netherlands
(A) δ13C and (B) δ15N isotope values in per mil (‰). The raincloud (half-density) plot (top) enhances the traditional boxplot (middle) by highlighting multiple modalities (an indicator that groups may exist) and how distributions vary compared to the median and inner-quartile range. The half-dotplot (lowest), which is similar to a histogram, indicates the number of samples (number of dots) in each bin.
Human dental elements
Dental enamel
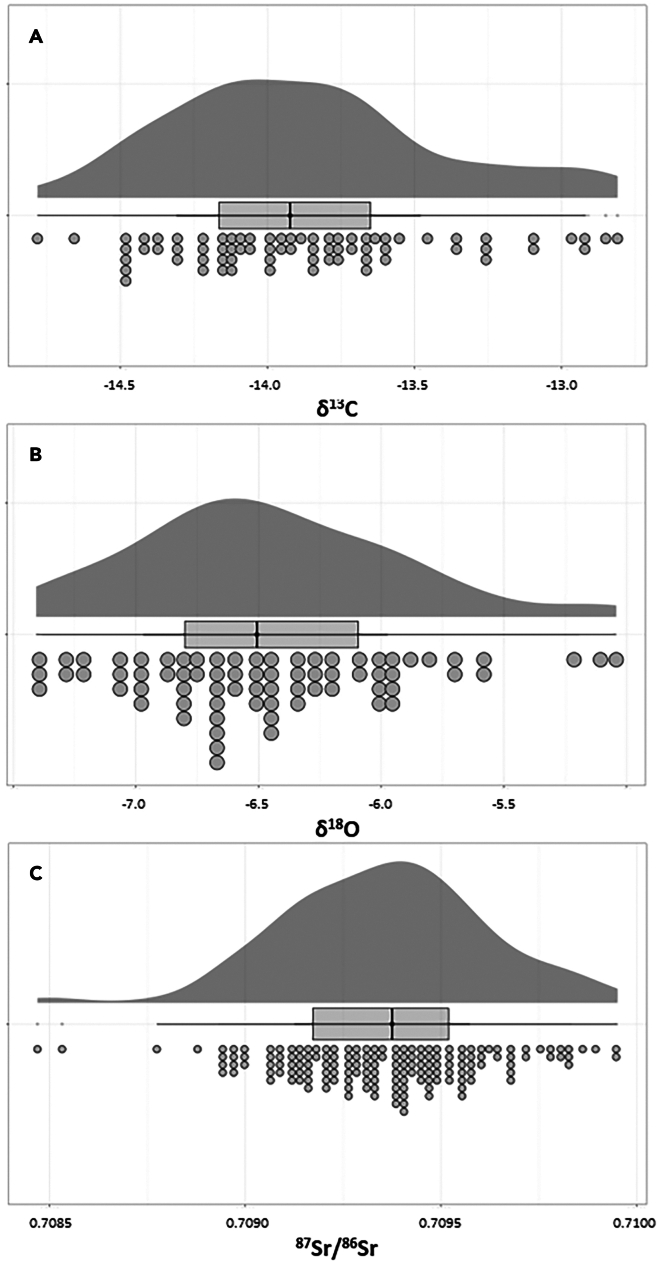
A total of 73 dental enamel samples were analyzed for O and C isotopes. Furthermore, we added six individuals to the previously published 87Sr/86Sr dataset,14 which now consists of 159 data points (Supplementary Data). The δ18O values recorded range from −7.4‰ to −5.0‰, with the mean being −6.5‰ ± 0.5‰ (1σ). The average δ13C isotope value is −13.9‰ ± 0.5‰ (1σ), with the minimum being −14.8‰ and the maximum being −12.8‰. The 87Sr/86Sr ranges from 0.70896 to 0.70942, averaging 0.70919 ± 0.00024 (1σ, Table 1). A visual representation of the distribution of these isotope values can be found in Figure 2.
Figure 2.
Raincloud plot of the isotope values and ratios found in human dental enamel from the Netherlands
(A) δ13C and (B) δ18O isotope values in per mil (‰), and (C) 87Sr/86Sr. The raincloud (half-density) plot (top) enhances the traditional boxplot (middle) by highlighting multiple modalities (an indicator that groups may exist) and how distributions vary compared to the median and inner-quartile range. The half-dotplot (lowest), which is similar to a histogram, indicates the number of samples (number of dots) in each bin.
Dental collagen
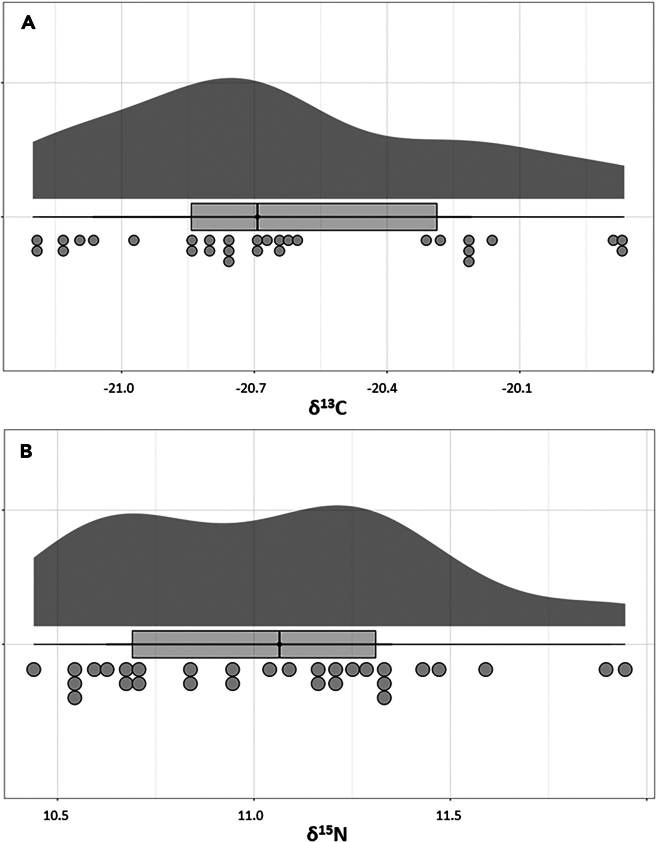
A total of 38 tooth dentine collagen samples were analyzed for their δ13C and δ15N isotope values (Supplementary Data). The δ13C values average −20.7‰ ± 0.4‰ (1σ), with the minimum being −22.0‰ and the maximum being −19.9‰. The δ15N values recorded range from 10.2‰ to 11.9‰, with the mean being 11.0‰ ± 0.4‰ (1σ, Table 1). A visual representation of the distribution of these isotope data can be found in Figure 3.
Figure 3.
Raincloud plot of the isotope values found in human tooth collagen from the Netherlands
(A) δ13C and (B) δ15N isotope values in per mil (‰). The raincloud (half-density) plot (top) enhances the traditional boxplot (middle) by highlighting multiple modalities (an indicator that groups may exist) and how distributions vary compared to the median and inner-quartile range. The half-dotplot (lowest), which is similar to a histogram, indicates the number of samples (number of dots) in each bin.
Tap water
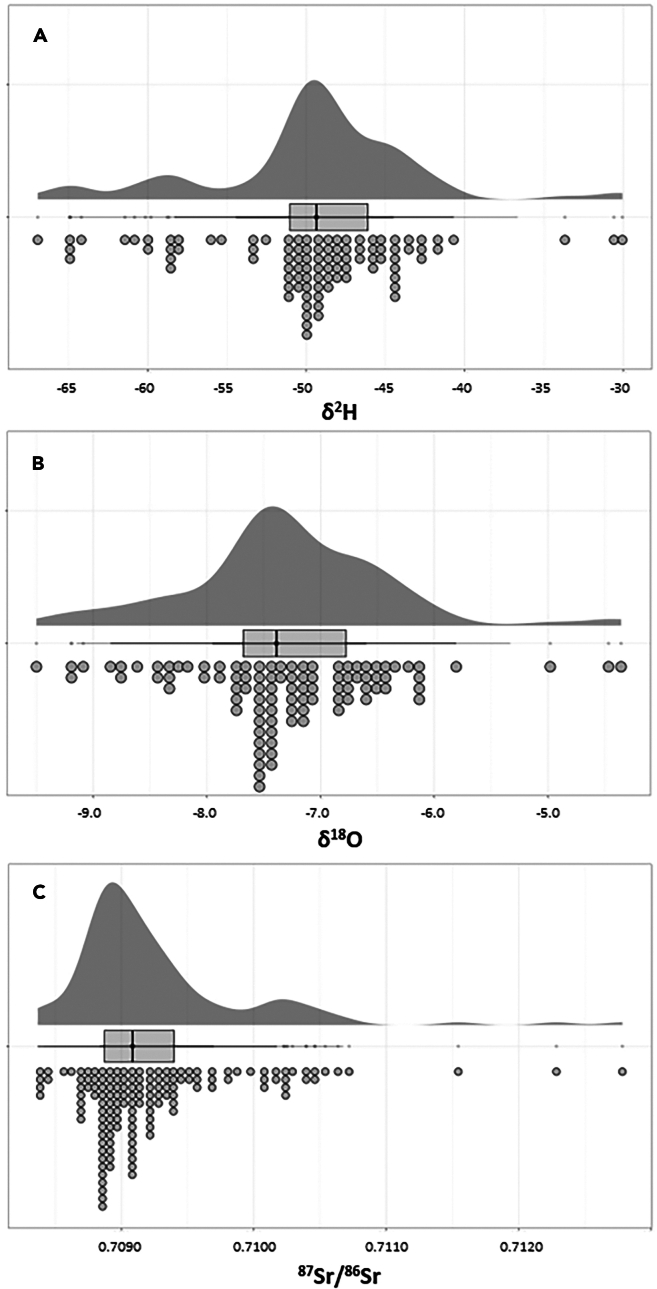
Of a total of 143 tap water samples, 98 samples were analyzed for their δ18O and δ2H isotope values. All 143 samples were previously analyzed for their Sr isotope ratios (Data S1).52 The δ18O values range from −9.5‰ to −4.4‰, with the mean being −7.3‰ ± 0.9‰ (1σ). The δ2H isotope values average −49.7‰ ± 6.5‰ (1σ), with the minimum being −66.9‰ and the maximum −30.1‰. The 87Sr/86Sr ranges from 0.70837 to 0.71278, averaging 0.70927 ± 0.00067 (1σ, Table 1). A visual representation of the distribution of these isotope values can be found in Figure 4.
Figure 4.
Raincloud plot of the isotope values and ratios found in tap water from the Netherlands
(A) δ2H and (B) δ18O isotope values in per mil (‰), and (C) 87Sr/86Sr. The raincloud (half-density) plot (top) enhances the traditional box-plot (middle) by highlighting multiple modalities (an indicator that groups may exist) and how distributions vary compared to the median and inner-quartile range. The half-dotplot (lowest), which is similar to a histogram, indicates the number of samples (number of dots) in each bin.
Discussion
Human hair
The hair keratin isotope data obtained in this study reflect the dietary intake in the months to years leading up to the donation in 2021. When comparing these data to older cold forensic cases, it is important to consider the sample date. Over time, dietary preferences and the availability of raw ingredients may have undergone shifts or alterations. Consequently, the Dutch hair C and N isotope data presented in this paper (collected in 2021) may not accurately represent the hair isotope values in significantly older cases. Furthermore, it is notable that the samples were analyzed in bulk, as described in the STAR Methods, which means that potential variations within the hair were not observable.15
Nitrogen isotope data
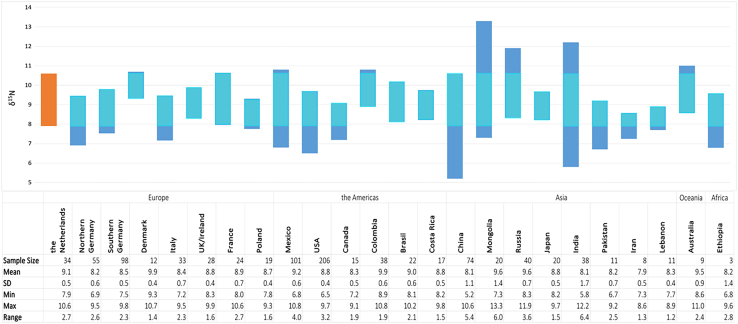
The collected hair keratin nitrogen isotope data from the Netherlands show a range of 2.2‰. This outcome may partially be attributed to the limited spatial diversity in the collection of samples, as most donors resided in the coastal provinces of Noord- and Zuid-Holland. Nevertheless, the overall isotopic variation may also be constrained owing to the widespread adoption of a dominant “supermarket-diet” and the substantial geographic expanse of the country. However, the range is comparable with the range observed in other countries (Figure 5). It is therefore likely that that amount of protein consumed is not dependent on food availability or geographical origin but varies greatly due to the personal preferences of the individual.
Figure 5.
Comparison of δ15N human hair values from various countries
Range of human hair δ15N values from the Netherlands (this study and previously published data41), and selected countries, such as Mexico,49 United States,53 Colombia,54 China,55 India,55 Mongolia,55 Pakistan.55 The data for all other countries are taken from the study by Lehn et al.56 All isotope values in per mil (‰). More reference data can be found elsewhere.9,57
The Dutch δ15N values are compared to published data from other countries in Figure 5. The descriptive statistics of all countries can be found in the Supplementary Data in tabular format. An additional six hair samples were incorporated in this discussion sampled in 2004 and 2013 from the Netherlands which were previously published,56 thereby expanding the Dutch dataset to a total of 34 data points. The δ15N values of human hair from the Netherlands fall within the range of values observed worldwide and, therefore, cannot be utilized to differentiate from the countries represented in this study.3,49,54,55,56 However, based on the current data, if δ15N values outside the range observed in the Netherlands are encountered in an unidentified individual, the Netherlands can be excluded as a possible source.
Carbon isotope data
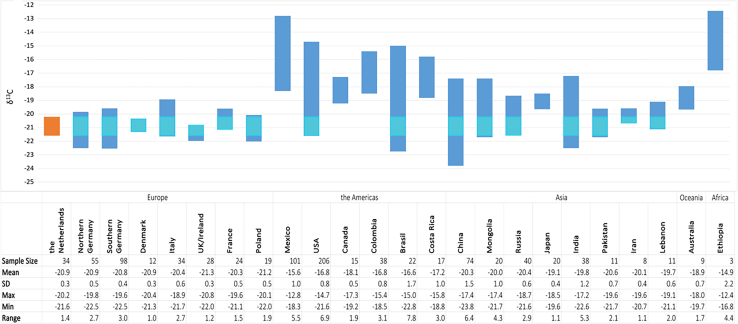
The δ13C variation observed in this study is narrow (1.2‰), akin to the nitrogen isotope data. The underlying factors contributing to this limited variation are likely consistent with those mentioned previously. In Figure 6 and the Supplementary data (tabular format), the Dutch dataset is compared to available data from various countries worldwide. Unlike the nitrogen isotope data, distinct disparities are evident between European countries and other regions globally. The δ13C values have less variance and are lower than the countries in the Americas and on the Asian and African continents. The available European data are similar, with the Netherlands occupying an intermediate position. Therefore, similarly to the δ15N data, it is not feasible to identify an unknown individual as Dutch solely based on δ13C values. However, it is possible to exclude the Netherlands as a plausible region of origin if the data deviate from the recorded values from the Netherlands.
Figure 6.
Comparison of δ13C human hair values from various countries
Range of human hair δ13C values from the Netherlands (this study and previously published data41), and selected countries, such as Mexico,49 United States,53 Colombia,54 China,55 India,55 Mongolia,55 Pakistan.55 The data for all other countries are taken from the study by Lehn et al.56 All isotope values in per mil (‰).
Human dental elements
Dental elements record the dietary intake of an individual during the early years of life until adolescence. Depending on the dental element, enamel of the permanent dentition is formed between birth and ca. the age of 16.32,58 Formation of the primary root dentine of the third molar starts at an approximate age of 15.5 years and is commonly completed by the age of 25.32,59 However, the continuous formation of secondary dentine throughout the lifespan of the tooth allows for the recording of an ongoing isotope signature of the food consumed in later stages of life.32 Because the roots in this study are bulk analyzed, the data presented here are dominated by the primary dentine recorded during youth and adolescence. The contribution of the continuously forming secondary dentine is negligible due to the low quantities formed.60 Consequently, the generated data may represent a diet consumed several decades ago, depending on the age of the donor. As a result of changing dietary habits over time, the Dutch data presented in this paper may not accurately reflect the expected C and N isotope data in significantly older or more recent cases.
Dental enamel
The overall variation in apatite δ18O and δ13C is limited (2.4‰ and 2.0‰, respectively) and barely larger than the observed intra-dental variation in modern Dutch individuals (2.0‰).17 The variation in 87Sr/86Sr data is also limited (0.00046) and exceeds the observed intra-dental variation of 0.0002 by a factor of 2.17 Moreover, all data are within the earlier published range that is considered indicative for the modern Dutch population (0.7088–0.7099).18 Unfortunately, very limited data for European comparison are available. The 87Sr/86Sr recorded in Dutch teeth overlaps with many other countries, although not with Mexico and Brazil (Table 2), underlining the need for a multi-isotope approach. The Dutch oxygen isotope data overlap with the Bulgarian, although modern human dental enamel δ18O reference data are even more scarce than that of strontium. The Dutch δ13C data fall within the range of available reference data, and therefore does not allow for distinction (Table 2). Using established conversion equations,61,62,63 the δ18OVPDB data can be converted to δ18OVSMOW and δ18ODW to allow comparisons with tap water or environmental water data.61,62,63 The converted δ18OVSMOW ranges between 23.3‰ and 25.7‰ and the δ18ODW between −11.6‰ and −7.8‰. The human δ18ODW data exceed the minimum δ18ODW observed in the tap water samples (−9.5‰, see “Tap water”), underlining the difficulty to use conversation equations as a manner to compare primary dietary sources (e.g., drinking water) to human tissues data.
Table 2.
Strontium ratios and oxygen and carbon isotope values from selected countries for comparison of dental enamel
| 87Sr/86Sr | δ18O (‰) | δ13C (‰) | |
|---|---|---|---|
| Brasil64 | 0.71015 to 0.71566 (n = 75) | – | −16.66 to −13.40 (n = 75) |
| Vietnam65 | 0.70805 to 0.71823 (n = 23) | −10.61 to −5.04 (n = 48) | −17.25 to −6.32 (n = 48) |
| USA65 | 0.70797 to 0.71061 (n = 26) | −12.57 to −3.75 (n = 158) | −12.88 to −7.77 (n = 158) |
| Mexico66 | 0.7044 to 0.7064 (n = 19) | – | – |
| Norway67 | 0.70773 to 0.71769 (n = 8) | – | – |
| Bulgaria68 | 0.70833 to 0.70908 (n = 15) | −7.6 to −5.4 (n = 12) | −12.7 to −10.6 (n = 12) |
| Germanya | 0.70867 to 0.71046 (n = 7) | – | – |
Unpublished data Vrije Universiteit Amsterdam.
Dentine collagen
The δ15N and δ13C values in the dentine-derived collagen samples show limited variability (1.7‰ and 2.1‰, respectively). In accordance with previously published data, which indicated that bone collagen was relatively enriched compared to the same individual’s hair keratin (0.86‰–1.50‰ in nitrogen and 0.6‰–1.4‰ in carbon),56,69 this study found that the tooth dentine collagen was enriched relative to the hair keratin (average 1.8‰ in nitrogen and 0.2‰ in carbon). However, it must be noted that the number of samples in the datasets is different, and the data do not come from the same individual. This may account for the relatively significant difference in δ15N and limited enrichment in δ13C. Additional research is needed to investigate the use of hair keratin isotope data as a proxy for bone or dental collagen.
Tap water
The first oxygen and hydrogen isotope data of Dutch tap water show a greater variation in δ18O compared to the human enamel data (5.1‰ vs. 2.4‰), and a range of 36.8‰ in δ2H (−66.9‰ and −30.1‰). The variation in δ18O can be explained by the rather complex tap water system in the Netherlands, using different environmental and natural resources (precipitation, ground water, river water, etc.).18 The range in δ2H is large (36.8‰), considering the proximity to the sea and limited elevation range, which are commonly considered the driving forces in the variability of stable light isotope values. For comparison, the range in δ2H in Mexico, a country 47 times the size of the Netherlands with elevation ranging up to 5,675 m, is only twice as large, 82.4‰.70 The range in the Netherlands is in part controlled by the origin of river water in Germany and the Alps.
Limitations of the study
One notable limitation of this research, as well as many previous studies, is that the donated hair samples used in the analysis are already relatively “clean” compared to hair found in forensic contexts, which may be contaminated by decomposition fluids, rain, soil, or other external influences. However, it is important to acknowledge that even in “clean” hair samples, other exogenous factors such as the use of shampoo, conditioner, relaxing agents, etc., can still influence the biogenic isotope composition and should therefore not be disregarded. Previous research, however, provided evidence that the carbon and nitrogen isotope systems were relatively robust, resulting in limited diagenetic alterations in hair δ13C and δ15N over time and providing more reliable data than H-O-Sr isotope ratios.71 Nevertheless, further (experimental) research is necessary to explore the recovery of endogenous isotope signatures from both “dirty” (soiled) and “clean” hair samples, considering various isotope systems.71,72 While this research has contributed to the expanding database of isotope values and ratios, albeit with a restricted number of samples, it is important to note that the spatial coverage of all human tissue data is still limited.
The analysis of samples in bulk leaves the intra-individual variation as an unexplored aspect in forensic isotope research. This highlights the need for further investigation in this area. Furthermore, this study handled the data as bulk data, without taking the metadata from the questionnaires into account. To better understand deviant data points with regards to dietary habits, pathological conditions, cultural heritage, etc., and to increase the interpretational accuracy of the data, comparative analyses of metadata and isotope values should be conducted in the future.
Additionally, this study did not incorporate Pb isotope data, which has demonstrated significant potential in forensic isotope research for determining geographic origin in cases of unidentified deaths (unpublished data Vrije Universiteit Amsterdam). To effectively utilize Pb isotope research on a broader scale in the future, it is crucial to expand the reference dataset with relevant human data.
The ability to compare the Dutch dental enamel and dentine collagen isotope data to other country’s data is limited due to sheer lack of availability. It is thus of essence to increase the publicly available data of identified modern human tissues, especially dental elements, and bones. Examples of successful sample and data acquisition are IDIS (collaboration of the NFI and the Vrije Universiteit Amsterdam) and the currently ongoing Project FIND-EM in the United States.
Furthermore, this study showed that it remains difficult to use tap water data for human relocation. As previously published by Kootker et al., tap water has a limited impact on isotope intake in Dutch individuals. This is likely due to an increasing globalization of food distribution, making tap water an unsuitable reference material for forensic investigations of isotopes in modern dental enamel within the Netherlands.
Conclusions
The accurate determination of an individual’s geographic origin is a fundamental aspect of forensic investigations. Isotope analysis potentially plays a crucial role in this process by providing information about an individual’s dietary patterns, environmental exposure, and mobility history. By incorporating the reference isotope values and ratios specific to the Dutch inhabitants and environment, the accuracy and reliability of isotope analysis in forensic investigations within the Netherlands can be significantly improved. Specifically, it enhances our ability to distinguish between individuals originating from different geographic areas, which is crucial for narrowing down the pool of potential matches in unidentified cases. In this publication, the data of 28 hair (for nitrogen and carbon), 159 enamel (for strontium, of which 73 also for oxygen and carbon), 38 dentine, and 143 tap water (for strontium, of which 98 also for oxygen and hydrogen) samples were presented and analyzed. Furthermore, if possible, they were compared to available data from other countries.
The δ15N values of human hair from the Netherlands fall within the range of values observed worldwide and, therefore, cannot be utilized to differentiate from the countries represented in this study. Unlike the nitrogen isotope data, distinct disparities in the hair carbon isotope data are evident between European countries and other regions globally. Yet, it is not feasible to identify an unknown individual as Dutch solely based on δ13C values. However, it is possible to exclude the Netherlands as a plausible region of origin if the data deviate from the recorded values from the Netherlands. Furthermore, the human dental enamel δ18ODW data exceed the minimum δ18ODW observed in the tap water samples (−9.5‰, see “Tap water”), underlining the difficulty to use conversation equations as a manner to compare primary dietary sources (e.g., drinking water) to human tissues data.
The task of comparing Dutch Sr-O-C-N isotope data from dental enamel and dentine collagen to that of other (European) nations has encountered significant challenges due to the limited availability of publicly accessible data. The scarcity of European reference data further hampers the possibility of conducting a meaningful comparison specifically involving the Dutch dental enamel 87Sr/86Sr. The same limitation applies to the tap water δ2H data. Nevertheless, these data are of utmost significance as they establish a foundation for future comparisons and enable researchers to include or exclude the Netherlands as a potential country of origin.
The findings of this study make a significant contribution to the growing database of isotope values and ratios worldwide. This expanded dataset holds immense value in the context of forensic investigations, particularly in enhancing our understanding of modern human isotopic signatures within the Netherlands. Further, it strengthens our ability to determine the geographic origin of unknown individuals, facilitating victim identification efforts and contributing to the advancement of forensic science in the Netherlands.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human Hair | This study | 1 |
| Human Hair | This study | 2 |
| Human Hair | This study | 3 |
| Human Hair | This study | 4 |
| Human Hair | This study | 10 |
| Human Hair | This study | 11 |
| Human Hair | This study | 12 |
| Human Hair | This study | 15 |
| Human Hair | This study | 18 |
| Human Hair | This study | 19 |
| Human Hair | This study | 20 |
| Human Hair | This study | 21 |
| Human Hair | This study | 23 |
| Human Hair | This study | 24 |
| Human Hair | This study | 25 |
| Human Hair | This study | 26 |
| Human Hair | This study | 28 |
| Human Hair | This study | 29 |
| Human Hair | This study | 30 |
| Human Hair | This study | 31 |
| Human Hair | This study | 32 |
| Human Hair | This study | 35 |
| Human Hair | This study | 39 |
| Human Hair | This study | 41 |
| Human Hair | This study | 42 |
| Human Hair | This study | 43 |
| Human Hair | This study | 44 |
| Human Hair | This study | 45G |
| Human Hair | This study | 45B |
| Human Hair | This study | 45M |
| Human Tooth | Kootker et al.18 | Breda 13 |
| Human Tooth | Kootker et al.18 | Breda 1 |
| Human Tooth | Kootker et al.18 | Helmond 2 |
| Human Tooth | Kootker et al.18 | Folter 3 |
| Human Tooth | Kootker et al.18 | Zwartsluis 4 |
| Human Tooth | Kootker et al.18 | Folter 7 |
| Human Tooth | Kootker et al.18 | Folter 8 |
| Human Tooth | Kootker et al.18 | Helmond 11 |
| Human Tooth | Kootker et al.18 | Strijen 15 |
| Human Tooth | Kootker et al.18 | Erasmus 1 |
| Human Tooth | Kootker et al.18 | Helmond 14 |
| Human Tooth | Kootker et al.18 | Strijen 14 |
| Human Tooth | Kootker et al.18 | Z-Heerlen 2 |
| Human Tooth | Kootker et al.18 | Strijen 17 |
| Human Tooth | Kootker et al.18 | Helmond 16 |
| Human Tooth | Kootker et al.18 | Zwartsluis 8 |
| Human Tooth | Kootker et al.18 | Zwartsluis 3 |
| Human Tooth | Kootker et al.18 | Folter 4 |
| Human Tooth | Kootker et al.18 | Folter 5 |
| Human Tooth | Kootker et al.18 | Strijen 6 |
| Human Tooth | Kootker et al.18 | Breda 14 |
| Human Tooth | Kootker et al.18 | Twente 4 |
| Human Tooth | Kootker et al.18 | Helmond 5 |
| Human Tooth | Kootker et al.18 | Zwartsluis 2 |
| Human Tooth | Kootker et al.18 | Veldhoven 8 |
| Human Tooth | Kootker et al.18 | 's-Hertogenbosch 15 |
| Human Tooth | Kootker et al.18 | Folter 1 |
| Human Tooth | Kootker et al.18 | Breda 3 |
| Human Tooth | Kootker et al.18 | Z.Heerlen 8 |
| Human Tooth | Kootker et al.18 | Erasmus 11 |
| Human Tooth | Kootker et al.18 | 's-Hertogenbosch 17 |
| Human Tooth | Kootker et al.18 | Maastricht 16 |
| Human Tooth | Kootker et al.18 | Sneek 12 |
| Human Tooth | Kootker et al.18 | Breda 4 |
| Human Tooth | Kootker et al.18 | Erasmus 13 |
| Human Tooth | Kootker et al.18 | Strijen 16 |
| Human Tooth | Kootker et al.18 | Folter 2 |
| Human Tooth | Kootker et al.18 | Zwartsluis 6 |
| Human Tooth | Kootker et al.18 | Folter 6 |
| Human Tooth | Kootker et al.18 | Zeeland 8 |
| Human Tooth | Kootker et al.18 | Helmond 3 |
| Human Tooth | Kootker et al.18 | Helmond 8 |
| Human Tooth | Kootker et al.18 | Veldhoven 9 |
| Human Tooth | Kootker et al.18 | Friesland 14 |
| Human Tooth | Kootker et al.18 | Helmond 6 |
| Human Tooth | Kootker et al.18 | Drenthe 16 |
| Human Tooth | Kootker et al.18 | Geldermalsen 8 |
| Human Tooth | Kootker et al.18 | Maastricht 15 |
| Human Tooth | Kootker et al.18 | Geldermalsen 5 |
| Human Tooth | Kootker et al.18 | Breda 18 |
| Human Tooth | Kootker et al.18 | Z.Heerlen 7 |
| Human Tooth | Kootker et al.18 | Steenwijk 7 |
| Human Tooth | Kootker et al.18 | Breda 2 |
| Human Tooth | Kootker et al.18 | Helmond 10 |
| Human Tooth | Kootker et al.18 | 's-Hertogenbosch 18 |
| Human Tooth | Kootker et al.18 | 's-Hertogenbosch 7 |
| Human Tooth | Kootker et al.18 | Maastricht 3 |
| Human Tooth | Kootker et al.18 | Maastricht 17 |
| Human Tooth | Kootker et al.18 | Geldermalsen 1 |
| Human Tooth | Kootker et al.18 | Erasmus 3 |
| Human Tooth | Kootker et al.18 | Geldermalsen 4 |
| Human Tooth | Kootker et al.18 | Steenwijk 1 |
| Human Tooth | Kootker et al.18 | Sneek 3 |
| Human Tooth | Kootker et al.18 | Zwartsluis 5 |
| Human Tooth | Kootker et al.18 | Heerlen 10 |
| Human Tooth | Kootker et al.18 | Friesland 18 |
| Human Tooth | Kootker et al.18 | Strijen 10 |
| Human Tooth | Kootker et al.18 | 's-Hertogenbosch 2 |
| Human Tooth | Kootker et al.18 | Breda 8 |
| Human Tooth | Kootker et al.18 | Veldhoven 16 |
| Human Tooth | Kootker et al.18 | Helmond 7 |
| Human Tooth | Kootker et al.18 | Geldermalsen 7 |
| Human Tooth | Kootker et al.18 | Maastricht 6 |
| Human Tooth | Kootker et al.18 | Sneek 1 |
| Human Tooth | Kootker et al.18 | Friesland 10 |
| Human Tooth | Kootker et al.18 | Helmond 15 |
| Human Tooth | Kootker et al.18 | Helmond 13 |
| Human Tooth | Kootker et al.18 | Erasmus 14 |
| Human Tooth | this publication | VU_DB |
| Human Tooth | Kootker et al.18 | Sneek 6 |
| Human Tooth | Kootker et al.18 | Zwartsluis 1 |
| Human Tooth | Font et al.3,4 | IDID_013NL |
| Human Tooth | Font et al.3,4 | IDID_014NL |
| Human Tooth | Font et al.3,4 | IDID_015NL |
| Human Tooth | Font et al.3,4 | IDID_016NL |
| Human Tooth | Font et al.3,4 | IDIS_001NL |
| Human Tooth | Font et al.3,4 | IDIS_002NL |
| Human Tooth | Font et al.3,4 | IDIS_003NL |
| Human Tooth | Font et al.3,4 | IDIS_004NL |
| Human Tooth | Font et al.3,4 | IDIS_005NL |
| Human Tooth | Font et al.3,4 | IDIS_006NL |
| Human Tooth | Font et al.3,4 | IDIS_007NL |
| Human Tooth | Font et al.3,4 | IDIS_008NL |
| Human Tooth | Font et al.3,4 | IDIS_009NL |
| Human Tooth | Font et al.3,4 | IDIS_010NL |
| Human Tooth | Font et al.3,4 | IDIS_011NL |
| Human Tooth | Font et al.3,4 | IDIS_012NL |
| Human Tooth | Font et al.3,4 | IDIS_017NL |
| Human Tooth | Font et al.3,4 | IDIS_018NL |
| Human Tooth | Font et al.3,4 | IDIS_019NL |
| Human Tooth | Font et al.3,4 | IDIS_020NL |
| Human Tooth | Font et al.3,4 | IDIS_021NL |
| Human Tooth | Font et al.3,4 | IDIS_022NL |
| Human Tooth | Font et al.3,4 | IDIS_023NL |
| Human Tooth | Font et al.3,4 | IDIS_024NL |
| Human Tooth | Font et al.3,4 | IDIS_025NL |
| Human Tooth | Font et al.3,4 | IDIS_026NL |
| Human Tooth | Font et al.3,4 | IDIS_027NL |
| Human Tooth | Font et al.3,4 | IDIS_028NL |
| Human Tooth | Font et al.3,4 | IDIS_029NL |
| Human Tooth | Font et al.3,4 | IDIS_030NL |
| Human Tooth | Kootker et al.18 | Almelo 1 |
| Human Tooth | Kootker et al.18 | Folter 2-C |
| Human Tooth | Kootker et al.18 | Heerlen 5 |
| Human Tooth | Kootker et al.18 | Sneek 11 |
| Human Tooth | Kootker et al.18 | Sneek 13 |
| Human Tooth | Kootker et al.18 | Sneek 14 |
| Human Tooth | Kootker et al.18 | Sneek 4 |
| Human Tooth | Kootker et al.18 | Steenwijk 4 |
| Human Tooth | Kootker et al.18 | Steenwijk 6 |
| Human Tooth | Kootker et al.18 | Strijen 3 |
| Human Tooth | Kootker et al.18 | Strijen 8 |
| Human Tooth | Kootker et al.18 | Zeeland 1 |
| Human Tooth | Kootker et al.18 | Zeeland 3 |
| Human Tooth | Kootker et al.18 | Zeeland 6 |
| Human Tooth | Plomp et al.73 | 28-R14a |
| Human Tooth | Plomp et al.73 | 29-R11 |
| Human Tooth | Plomp et al.73 | 30-R13 |
| Human Tooth | Plomp et al.73 | 34-R5 |
| Human Tooth | Plomp et al.73 | 35-R9 |
| Human Tooth | Plomp et al.73 | 36-F1 |
| Human Tooth | Plomp et al.73 | 37-F3 |
| Human Tooth | Plomp et al.73 | 38-F4 |
| Human Tooth | Plomp et al.73 | 39-F8 |
| Human Tooth | Plomp et al.73 | 40-F11 |
| Human Tooth | Plomp et al.73 | 41-F12 |
| Human Tooth | Plomp et al.73 | 42-F13 |
| Human Tooth | Plomp et al.73 | 43-R6 |
| Human Tooth | Plomp et al.73 | 44-M4 |
| Human Tooth | Plomp et al.73 | 45-M5 |
| Human Tooth | Plomp et al.73 | 47-M14 |
| Human Tooth | Plomp et al.73 | 48-ZH1 |
| Human Tooth | Plomp et al.73 | 49-ZH3 |
| Human Tooth | Plomp et al.73 | 50-ZH4 |
| Human Tooth | Plomp et al.73 | 51-ZH9 |
| Human Tooth | Plomp et al.17 | Drenthe-15 |
| Human Tooth | Plomp et al.17 | Drenthe-3 |
| Human Tooth | Plomp et al.17 | Friesland-6 |
| Human Tooth | Plomp et al.17 | Friesland-7 |
| Human Tooth | Plomp et al.17 | Limburg-6 |
| Human Tooth | Plomp et al.17 | Twente-1 |
| Human Tooth | Plomp et al.17 | Twente-2 |
| Human Tooth | Plomp et al.17 | Twente-6 |
| Human Tooth | Plomp et al.17 | Zuid Holland 13 |
| Human Tooth | this publication | AO |
| Human Tooth | this publication | JVDS |
| Human Tooth | this publication | SM |
| Human Tooth | this publication | VU_DB |
| Human Tooth | this publication | VU_JK |
| Tap Water | Kootker et al.18 | Tiel II |
| Tap Water | Kootker et al.18 | Gouda |
| Tap Water | Kootker et al.18 | Leiderdorp |
| Tap Water | Kootker et al.18 | Alphen aan de Rijn |
| Tap Water | Kootker et al.18 | Vianen |
| Tap Water | Kootker et al.18 | Enspijk |
| Tap Water | Kootker et al.18 | Ridderkerk |
| Tap Water | Kootker et al.18 | Schijndel |
| Tap Water | Kootker et al.18 | Zwolle |
| Tap Water | Kootker et al.18 | Utrecht Zuilen II |
| Tap Water | Kootker et al.18 | Serooskerke |
| Tap Water | Kootker et al.18 | Amstelveen |
| Tap Water | Kootker et al.18 | Heemstede |
| Tap Water | Kootker et al.18 | Haarlem |
| Tap Water | Kootker et al.18 | Ijsselstein |
| Tap Water | Kootker et al.18 | Aalsmeer |
| Tap Water | Kootker et al.18 | Vijlen |
| Tap Water | Kootker et al.18 | Eindhoven |
| Tap Water | Kootker et al.18 | Heiloo |
| Tap Water | Kootker et al.18 | Valkenburg (L) |
| Tap Water | Kootker et al.18 | Wageningen |
| Tap Water | Kootker et al.18 | Velp |
| Tap Water | Kootker et al.18 | Heerlen |
| Tap Water | Kootker et al.18 | Mechelen |
| Tap Water | Kootker et al.18 | Nijmegen II |
| Tap Water | Kootker et al.18 | Nijmegen |
| Tap Water | Kootker et al.18 | Maastricht |
| Tap Water | Kootker et al.18 | Echt |
| Tap Water | Kootker et al.18 | Arnhem Centrum |
| Tap Water | Kootker et al.18 | Hengelo |
| Tap Water | Kootker et al.18 | Ede |
| Tap Water | Kootker et al.18 | Ermelo |
| Tap Water | Kootker et al.18 | Kampen II |
| Tap Water | Kootker et al.18 | Almere Centrum |
| Tap Water | Kootker et al.18 | Borger |
| Tap Water | Kootker et al.18 | Susteren |
| Tap Water | Kootker et al.18 | Amersfoort |
| Tap Water | Kootker et al.18 | Arnhem Zuid |
| Tap Water | Kootker et al.18 | Doorn |
| Tap Water | Kootker et al.18 | Geleen |
| Tap Water | Kootker et al.18 | Almere |
| Tap Water | Kootker et al.18 | Nieuwegein |
| Tap Water | Kootker et al.18 | Bunnik |
| Tap Water | Kootker et al.18 | Apeldoorn |
| Tap Water | Kootker et al.18 | Hoenderloo |
| Tap Water | Kootker et al.18 | Sittard |
| Tap Water | Kootker et al.18 | Kampen |
| Tap Water | Kootker et al.18 | Brunssum |
| Tap Water | Kootker et al.18 | Utrecht Zuilen |
| Tap Water | Kootker et al.18 | Almelo |
| Tap Water | Kootker et al.18 | Utrecht |
| Tap Water | Kootker et al.18 | Enschede |
| Tap Water | Kootker et al.18 | Winterswijk |
| Tap Water | Kootker et al.18 | Haaksbergen |
| Tap Water | Kootker et al.18 | Veenendaal |
| Tap Water | Kootker et al.18 | Venlo |
| Tap Water | Kootker et al.18 | Steenwijk |
| Tap Water | Kootker et al.18 | Meppel |
| Tap Water | Kootker et al.18 | Den Helder |
| Tap Water | Kootker et al.18 | Oldenzaal |
| Tap Water | Kootker et al.18 | Horst |
| Tap Water | Kootker et al.18 | Lottum |
| Tap Water | Kootker et al.18 | Diever |
| Tap Water | Kootker et al.18 | Hilversum |
| Tap Water | Kootker et al.18 | Leiden |
| Tap Water | Kootker et al.18 | Gennep |
| Tap Water | Kootker et al.18 | Doetinchem |
| Tap Water | Kootker et al.18 | Appingedam |
| Tap Water | Kootker et al.18 | Dronten |
| Tap Water | Kootker et al.18 | Wassenaar |
| Tap Water | Kootker et al.18 | Rotterdam |
| Tap Water | Kootker et al.18 | Berkel |
| Tap Water | Kootker et al.18 | Breda |
| Tap Water | Kootker et al.18 | Tilburg |
| Tap Water | Kootker et al.18 | Oostvoorne |
| Tap Water | Kootker et al.18 | Hardenberg |
| Tap Water | Kootker et al.18 | Bergen op Zoom |
| Tap Water | Kootker et al.18 | Groningen II |
| Tap Water | Kootker et al.18 | America |
| Tap Water | Kootker et al.18 | Groningen |
| Tap Water | Kootker et al.18 | Middelburg |
| Tap Water | Kootker et al.18 | Hoorn |
| Tap Water | Kootker et al.18 | Poeldijk |
| Tap Water | Kootker et al.18 | Emmeloord |
| Tap Water | Kootker et al.18 | Zwijndrecht |
| Tap Water | Kootker et al.18 | Heerenveen |
| Tap Water | Kootker et al.18 | Achtmaal |
| Tap Water | Kootker et al.18 | Dalfsen |
| Tap Water | Kootker et al.18 | Roosendaal |
| Tap Water | Kootker et al.18 | Nuis |
| Tap Water | Kootker et al.18 | Delft |
| Tap Water | Kootker et al.18 | Den Andel |
| Tap Water | Kootker et al.18 | Hoogeveen |
| Tap Water | Kootker et al.18 | Venray |
| Tap Water | Kootker et al.18 | Roermond |
| Tap Water | Kootker et al.18 | Leeuwarden |
| Tap Water | Kootker et al.18 | Franeker |
| Tap Water | Kootker et al.18 | Harlingen |
| Tap Water | Kootker et al.18 | 's-Gravenhage |
| Tap Water | Kootker et al.18 | 's-Gravenhage II |
| Tap Water | Kootker et al.18 | Den Bosch II |
| Tap Water | Kootker et al.18 | Den Bosch |
| Tap Water | Kootker et al.18 | Texel |
| Tap Water | Font et al.3,4 | LF14 |
| Tap Water | Font et al.3,4 | LF52 |
| Tap Water | Font et al.3,4 | LF53 |
| Tap Water | Font et al.3,4 | LF20 |
| Tap Water | Kootker et al.18 | Boxmeer |
| Tap Water | Kootker et al.18 | Bredevoort |
| Tap Water | Kootker et al.18 | Breukelen |
| Tap Water | Kootker et al.18 | Delft II |
| Tap Water | Font et al.3,4 | LF18 |
| Tap Water | Kootker et al.18 | Drachten |
| Tap Water | Font et al.3,4 | LF51 |
| Tap Water | Kootker et al.18 | Emmen |
| Tap Water | Kootker et al.18 | Enkhuizen |
| Tap Water | Font et al.3,4 | LF19 |
| Tap Water | Font et al.3,4 | LF25 |
| Tap Water | Font et al.3,4 | LF50 |
| Tap Water | Font et al.3,4 | LF26 |
| Tap Water | Kootker et al.18 | Kerkrade |
| Tap Water | Font et al.3,4 | LF23 |
| Tap Water | Kootker et al.18 | Lelystad |
| Tap Water | Font et al.3,4 | LF49 |
| Tap Water | Kootker et al.18 | Norg II |
| Tap Water | Kootker et al.18 | Norg III |
| Tap Water | Kootker et al.18 | Ouddorp |
| Tap Water | Kootker et al.18 | Reuver |
| Tap Water | Font et al.3,4 | LF58 |
| Tap Water | Font et al.3,4 | LF55 |
| Tap Water | Font et al.3,4 | LF56 |
| Tap Water | Font et al.3,4 | LF57 |
| Tap Water | Kootker et al.18 | Soesterberg |
| Tap Water | Kootker et al.18 | Stadskanaal |
| Tap Water | Kootker et al.18 | Uden |
| Tap Water | Font et al.3,4 | LF84 |
| Tap Water | Font et al.3,4 | LF86 |
| Tap Water | Font et al.3,4 | LF85 |
| Tap Water | Kootker et al.18 | Velp NB |
| Tap Water | Kootker et al.18 | Vianen II |
| Tap Water | Kootker et al.18 | Wijk en Aalburg |
| Tap Water | Kootker et al.18 | Winschoten |
| Tap Water | Kootker et al.18 | Zwijndrecht II |
| Deposited data | ||
| Human Tooth Data | Figshare | Kootker et al.74 |
| Human Hair Data | Figshare | Ammer et al.75 |
| Tap Water Data | Figshare | Kootker et al.52 |
| Software and algorithms | ||
| R: A Language and Environment for Statistical Computing. http://www.r-project.org/ | ||
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact and corresponding authors Saskia Ammer (s.t.m.ammer@vu.nl).
Materials availability
This study did not generate new unique reagents. There are restrictions to the availability of reagents due to that the human materials in this study is in its entirety few, therefore considered to be a limited resource. The samples are physically stored at the Archaeological and Forensic sample preparation laboratory, Department of Earth Sciences, Vrije Universiteit Amsterdam, the Netherlands.
Data and code availability
-
•
De-identified human hair and tooth isotope data are deposited on Figshare and publicly available as of the date of publication.74,75
-
•
Tap water isotope data supporting the findings of this study are deposited on Figshare and publicly available as of the date of publication.52
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
The current modern-day human reference database of the Netherlands consists of human hair and dental elements. Tap water data is also included in this reference database as tap water is used for hydration, food preparation, as well as hygiene. A questionnaire was used associated with the collection of the hair samples (Questionnaire S1). The hair samples were collected between February and June of 2021. The dental elements were collected between 2010 and 2023. The tooth collection remains ongoing, we are thus able to continue adding to the database on a regular basis. Detailed information about the sampling locations of the tap water samples are published elsewhere.18
Human hair
For this study, a total of 28 hair samples were collected from anonymous donors. For the collection of the hair samples, written instructions were given to the donors how to cut and package the hair. The donors were also provided with a questionnaire asking questions about their diet and recent geographic whereabouts (Supplementary Data and Questionnaire S1). The samples were collected between February and June of 2021. The hair samples were wrapped in aluminium foil, placed in sealed envelopes and either collected in person or sent to the Vrije Universiteit Amsterdam. Any samples of individuals who spent time outside of the Netherlands during the respective growth period of the hair sample were excluded from this study. Hair samples were categorized based on colour using the Fischer-Saller scale (Figures S1 and S2).76 Hair type was determined using the method used by hairdressers established by Andre Walker, based on the morphology of the hair.77 Each sample was photographed and characterized for hair colour and type (Data S1, Figures S1 and S2). An overview of the answers to the questionnaire of the hair samples, as well as their hair colour and type categorization can be found in the Supplementary Data file. Human scalp hair grows at an average of 1 cm per month, and since the elemental exchange between hair and blood ceases when it dies off, it retains the biogenic and dietary information, preserving a longitudinal record of stable isotope signatures.15,78 In this study, the average represented timeframe is approximately six months, equalling ±6 cm hair length.
Human dental elements
The use of the human dental elements for scientific research was approved by the Medical Ethics Review Committee of the Amsterdam UMC, location VUmc. The samples were provided by dentist who extracted the elements, accompanied by a questionnaire about the dietary preferences and whereabouts of the donors during childhood (Questionnaire S2). None of the individuals relocated during the formation and mineralization period of the dental enamel and thus remained stationary in their respective place of residence in the Netherlands.
Tap water
For this study, a total of 98 tap water samples were analysed for their δ18O and δ2H isotope values to add to the existing database of 143 tap water samples analysed for 87Sr/86Sr.3,18 Analytical details for the Sr isotope analysis are provided in detail in Kootker et al.18
Method details
Human hair
Approximately 50-100 mg of hair from each sample was placed into 2 ml glass vials. The samples were cleaned by filling the vials with a two parts chloroform one part methanol solution and placed in an ultrasonic bath for 10 minutes. The methanol-chloroform mixture was then replaced with milli-Q water and placed back in the ultrasonic cleaner for 10 minutes. This process was performed three times before further leaching the samples with 0.01M HCL and Milli-Q again. The samples were then dried on a hotplate at 60°C and subsequently cut into circa 3mm pieces using scissors and stored in vials for sub sampling. Approximately 0.45 mg ± 10% of hair from all the samples were weighed into 5 x 9 mm tin capsules and weighed, along with standards of USGS40, USGS41, USGS42, and USGS43.
The δ15N and δ13C isotope values were measured using a Flash NC 1112series Elemental Analyser coupled to a Thermo Finnigan DeltaPlus XP Isotope Ratio Mass Spectrometer (IRMS) at the Earth Sciences Stable Isotope Laboratory, Vrije Universiteit Amsterdam. Instrument precision was better than 0.17‰ (1σ) for C-N isotopes based on replicate analysis of standard reference materials USGS42 (δ13C = –21.06 ± 0.13‰ and δ15N = 8.02 ± 0.15‰ (1σ, n =24) and USG43 (δ13C = –21.43 ± 0.10 ‰ and δ15N = 8.32 ± 0.17 ‰ (1σ, n =6)). The samples were calibrated using USGS40 and USGS41. The stable isotope results are expressed as δ (delta) values in per mil (‰) relative to Vienna Peedee Belemnite (VPDB) for δ13C and atmospheric nitrogen (AIR) for δ15N.
Human dental elements
Enamel powder was collected from the crown of the tooth using a Proxxon diamond tipped burr inside a fume hood, class 100. The burr was cleaned between each sample by being immersed in Milli-Q water, 10% hydrochloric acid (HCl), Milli-Q water again, and then ethanol. After removing the outermost surface of the enamel, circa 1-3 mg of dental enamel powder was collected using a diamond-tipped burr. The samples were taken from the mesial or distal lobe of either the buccal or lingual surface, depending on the physical quality of the molar and the presence of carious lesions.
For the δ18O analyses, circa 0.3 ± 10% mg enamel powder was weighed into a glass Exetainer® vial with screw-capped pierceable butyl rubber septa and transferred to the Stable Isotope laboratory at the Vrije Universiteit Amsterdam. The prepared vial was placed in a sample block interspaced with calibrations standards LSVEC, BCT (replacement for NBS19) and NBS18 and the in-house standards VICS for a linearity correction. The standard IAEA-603 is also analysed during each run as a control. After flushing the vials with helium, samples and standards were acidified with water-free H3PO4 (100%) at 45°C and allowed to react for 24 hours. The gas mixture was analysed using a Thermo Finnigan Delta plus IRMS with a GasBench II. The isotopic values are reported as δ (delta) values in ‰ units. Values were normalized to international standard IAEA-603 (δ18O = 2.34 ± 0.13‰ and δ13C = 2.52 ± 0.05‰ (1σ, n =16)) and are reported relative to the Vienna Peedee Belemnite (VPDB) standard.
For the 87Sr/86Sr analysis, all sample preparation and analyses were conducted at the Vrije Universiteit Amsterdam, the Netherlands. The dental enamel and tap water samples were sealed in acid-cleaned polyethylene Eppendorf centrifuge tubes and transported to the class 100 clean laboratory. The sample residues were dissolved in 500 μl 3M HNO3 for ion exchange chromatography. A detailed description of the Sr extraction/chromatography and the sample loading protocol are provided elsewhere.79 The strontium isotope compositions were measured on a ThermoFinnigan Triton Plus thermal ionisation mass spectrometer (TIMS). The ratios were determined using a static routine and were corrected for mass-fractionation to 86Sr/88Sr of 0.1194. The NBS987 standard gave a mean 87Sr/86Sr of 0.710257 ± 0.000006 (n = 113) during the period of analysis (2021). The current certified value of NBS987 is 0.71034 ± 0.00026 (certificate issue date 19 June 2007); event though accepted values vary significantly, between approximately 0.71024 to 0.710263.80,81 The procedural blanks contained on average 56 pg strontium (n = 9). The dataset was analysed using SPSS 25.0 (IBM SPSS Statistics for Macintosh, Armonk, IBM Corp.).
Thirty-eight of the teeth that have previously been analysed for their Sr isotope composition in dental enamel were now selected to create a first database of δ13C and δ15N isotope signatures in human tooth dentin. A Dremel diamond wheel was used to remove a portion of the dental root. The wheel was cleaned between each sample by being immersed in Milli-Q water, 10% HCl, Milli-Q water again, and then ethanol. The fragment was weighted using a digital balance and placed inside a 16 x 100 mm Elkay test tube (Elkay Labs, 2021). Subsequently 10 ml of 0.6M HCl was added to the test tubes to dissolve the bioapatite. The test tubes were stored in a fridge at 4°C for 48 hours. Subsequently, HCl was removed, and the firmness of the dentine was examined. If the dentine showed stiffness, then 10ml of 0.6M HCl was added back into the test tube and placed back in the fridge for another 48 hours. If the root had a sponge-like texture, then 9 ml of 0.01M HCl (pH3) was added using a dispenser and placed in an oven at 80°C for 48 hours. Following the removal of samples from the oven, the collagen was filtered using a 9 ml Ezee filter. The filtered collagen solution was then transferred into a weighed test tube, covered with parafilm, and stored in a freezer at -20°C. The collagen sample was then lyophilised for 48 hours, and reweighed. Approximately 0.50 mg ±10% of collagen was weighted into 5 x 9 mm tin capsules, along with control standards of USGS40, USGS41, USGS42, and USGS43.
The δ13C and δ15N values were measured using an elemental analyser (NCA500; ThermoQuest Italia, Rodana, Italy) coupled with an isotope ratio mass spectrometer (Delta Plus; Thermo-Quest Finnigan, Bremen, Germany) at the Earth Sciences Stable Isotope Laboratory, Vrije Universiteit Amsterdam. Instrument precision was better than 0.24 ‰ (1σ) for coupled C-N isotopes based on replicate analysis of standard reference materials USGS42 (δ13C = –21.13 ± 0.17 ‰ and δ15N = 8.04 ± 0.20 ‰ (1σ, n =5)) and USG43 (δ13C = –21.34 ± 0.14 ‰ and δ15N = 8.44 ± 0.24 ‰ (1σ, n =9)). The stable isotopes results are expressed as δ (delta) values in per mil (‰) relative to Vienna Peedee Belemnite (VPDB) for δ13C and atmospheric nitrogen for δ15N. The integrity of the collagen samples was assessed based on the atomic C:N ratio, the N and C abundances and collagen yield.82,83,84,85 The average C/N (mol/mol) was 3.54 ± 0.13 (1σ, n = 43).
Tap water
Tap water samples and standards were transferred to 2 ml crimp vials using a micro-pipet, and a clean tip for every sample and standard. Vials were then closed with a crimp cap. Samples were measured on a Picarro Inc L2140-i Wavelength-scanning cavity ring-down spectrometer at the Stable Isotope Laboratory, Department of Earth Sciences, Vrije Universiteit Amsterdam, in sets of 10, bracketed by four in-house laboratory standards. At least seven replicate analyses were performed per vial. The average and standard deviation of the last four injections were calculated for all samples and standards.
All standards are in-house water-standards that have been calibrated using the VSMOW and VSLAP (international water-standards from IAEA). Three of these standards are used for calibration of the samples. The fourth standard (KONA) is used as a control standard to determine the accuracy and precision of the measurement. The standard deviations of the isotopic values of KONA are <0.2 ‰ and <2 ‰ for δ18O and δ2H respectively. The calibrated values for KONA are –0.045 ‰ for δ18O and 0.6 ‰ for δ2H.
Quantification and statistical analysis
Mean and standard deviation were calculated from Excel for Sr, O, H, C, and N isotope data (see Table 1). Figures 1, 2, 3, and 4 were made using R. Figures 5 and 6 using Excel and Photoshop.
Acknowledgments
The Medical Ethics Review Committee of the Amsterdam UMC, location VUmc, is thanked for evaluating the sampling request (IDIS 2010/265). All anonymous donors (hair and teeth) are thanked for their contribution to this study. In addition, the following colleagues are acknowledged for the collection of water samples: Fraukje Brouwer, Margot Daleman, Joyce van Dijk, Maaike de Haas, Kinie Esser, Anja Fisher, Michael Gress, Maarten Groenendijk, Stijn Heeren, Alice Knaf, Jason Laffoon, Stefanie Luginbuehl, Coen Nienaber, Godfried Scheijvens, Richard Smeets, Ward Teertstra, Vincent van der Veen, Bas van der Wagt, and Jan Wijbrans.
Author contributions
S.T.M.A.: conceptualization, methodology, supervision, validation, formal analysis, investigation, data curation, writing – original draft, review and editing, and visualization. NR.: data collection, formal analysis, investigation, and writing – review and editing. G.R.D.: supervision, funding acquisition, and writing – review and editing. A.C.v.A.: supervision, funding acquisition, and writing – review and editing. S.J.A.V.-W.: formal analysis and writing – review and editing. L.M.K.: conceptualization, methodology, supervision, validation, formal analysis, investigation, data curation, project administration, writing – original draft, and review and editing.
Declaration of interests
The authors declare no competing interests.
Published: March 27, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109561.
Supplemental information
References
- 1.Kahana T., Hiss J. Identification of human remains: forensic radiology. J. Clin. Forensic Med. 1997;4:7–15. doi: 10.1016/S1353-1131(97)90002-X. [DOI] [PubMed] [Google Scholar]
- 2.INTERPOL Fact Sheet - Disaster victim identification. 2018. https://www.interpol.int/How-we-work/Forensics/Disaster-Victim-Identification-DVI
- 3.Font L., Jonker G., van Aalderen P.A., Schiltmans E.F., Davies G.R. Provenancing of unidentified World War II casualties: Application of strontium and oxygen isotope analysis in tooth enamel. Sci. Justice. 2015;55:10–17. doi: 10.1016/j.scijus.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Font L., Jonker G., van Aalderen P.A., Schiltmans E.F., Davies G.R. Addendum to “Provenancing of unidentified World War II casualties: Application of strontium and oxygen isotope analysis in tooth enamel” [Sci. Justice 55 (2015) 10–17] Sci. Justice. 2015;55:526. doi: 10.1016/j.scijus.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Augenstein W., Fraser I. Forensic isotope analysis leads to identification of a mutilated murder victim. Sci. Justice. 2008;48:153–159. doi: 10.1016/j.scijus.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Lehn C., Kalbhenn E.M., Rossmann A., Graw M. Revealing details of stays abroad by sequential stable isotope analyses along human hair strands. Int. J. Legal Med. 2019;133:935–947. doi: 10.1007/s00414-018-1866-9. [DOI] [PubMed] [Google Scholar]
- 7.Katzenberg M.A., Krouse H.R. Application of Stable Isotope Variation in Human Tissues to Problems in Identification. J. Can. Soc. Forensic. Sci. 1989;22:7–19. doi: 10.1080/00085030.1989.10757414. [DOI] [Google Scholar]
- 8.Nakamura K., Schoeller D.A., Winkler F.J., Schmidt H.L. Geographical variations in the carbon isotope composition of the diet and hair in contemporary man. Biomed. Mass Spectrom. 1982;9:390–394. doi: 10.1002/bms.1200090906. [DOI] [PubMed] [Google Scholar]
- 9.Lehn C., Rossmann A., Graw M., Davies G.R. Identification of a female murder victim found in Burgenland, Austria in 1993. Forensic Sci. Res. 2022;7:308–318. doi: 10.1080/20961790.2021.1924425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehn C., Graw M. Identifizierung einer skelettierten “Kofferleiche“ aus Berlin. Rechtsmedizin. 2016;26:429–435. doi: 10.1007/s00194-016-0091-4. [DOI] [Google Scholar]
- 11.Rauch E., Rummel S., Lehn C., Büttner A. Origin assignment of unidentified corpses by use of stable isotope ratios of light (bio-) and heavy (geo-) elements—A case report. Forensic Sci. Int. 2007;168:215–218. doi: 10.1016/j.forsciint.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Bataille C.P., Ammer S.T.M., Bhuiyan S., Chartrand M.M.G., St-Jean G., Bowen G.J. Multi-isotopes in human hair: A tool to initiate cross-border collaboration in international cold-cases. PLoS One. 2022;17 doi: 10.1371/journal.pone.0275902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesson L.A., Tipple B.J., Youmans L.V., O’Brien M.A., Harmon M.M. In: New Perspectives in Forensic Human Skeletal Identification. Latham K.E., Bartelink E.J., Finnegan M., editors. Academic Press; 2018. Chapter 14 - Forensic Identification of Human Skeletal Remains Using Isotopes: A Brief History of Applications From Archaeological Dig Sites to Modern Crime Scenes; pp. 157–173. [DOI] [Google Scholar]
- 14.Bartelink E.J., Chesson L.A. Recent applications of isotope analysis to forensic anthropology. Forensic Sci. Res. 2019;4:29–44. doi: 10.1080/20961790.2018.1549527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Font L., van der Peijl G., van Wetten I., Vroon P., van der Wagt B., Davies G. Strontium and lead isotope ratios in human hair: investigating a potential tool for determining recent human geographical movements. J. Anal. At. Spectrom. 2012;27:719–732. doi: 10.1039/c2ja10361c. [DOI] [Google Scholar]
- 16.Rodiouchkina K., Rodushkin I., Goderis S., Vanhaecke F. Longitudinal isotope ratio variations in human hair and nails. Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.152059. [DOI] [PubMed] [Google Scholar]
- 17.Plomp E., von Holstein I.C.C., Kootker L.M., Verdegaal-Warmerdam S.J.A., Forouzanfar T., Davies G.R. Strontium, oxygen, and carbon isotope variation in modern human dental enamel. Am. J. Phys. Anthropol. 2020;172:586–604. doi: 10.1002/ajpa.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kootker L.M., Plomp E., Ammer S.T.M., Hoogland V., Davies G.R. Spatial patterns in 87Sr/86Sr ratios in modern human dental enamel and tap water from the Netherlands: Implications for forensic provenancing. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138992. [DOI] [PubMed] [Google Scholar]
- 19.Pye K. Isotope and Trace Element Analysis of Human Teeth and Bones for Forensic Purposes. Geol. Soc. Spec. Publ. 2004;232:215–236. [Google Scholar]
- 20.Ueda M., Bell L.S. Paired stable carbon and oxygen isotope analyses of human enamel for forensic human geolocation: An exploratory study. J. Forensic Sci. 2023;68:382–398. doi: 10.1111/1556-4029.15212. [DOI] [PubMed] [Google Scholar]
- 21.Jaouen K., Szpak P., Richards M.P. Zinc Isotope Ratios as Indicators of Diet and Trophic Level in Arctic Marine Mammals. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu N.-C., Henderson G.M., Belshaw N.S., Hedges R.E. Establishing the potential of Ca isotopes as proxy for consumption of dairy products. Appl. Geochem. 2006;21:1656–1667. doi: 10.1016/j.apgeochem.2006.07.003. [DOI] [Google Scholar]
- 23.Lewis J., Pike A.W.G., Coath C.D., Evershed R.P. Strontium concentration, radiogenic (87Sr/86Sr) and stable (δ88Sr) strontium isotope systematics in a controlled feeding study. STAR: Sci. Technol. Archaeol. Res. 2017;3:45–57. doi: 10.1080/20548923.2017.1303124. [DOI] [Google Scholar]
- 24.Tipple B.J., Valenzuela L.O., Ehleringer J.R. Strontium isotope ratios of human hair record intra-city variations in tap water source. Sci. Rep. 2018;8:3334. doi: 10.1038/s41598-018-21359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeder H.A., Tipton I.H., Nason A.P. Trace metals in man: strontium and barium. J. Chronic Dis. 1972;25:491–517. doi: 10.1016/0021-9681(72)90150-6. [DOI] [PubMed] [Google Scholar]
- 26.Ericson J.E. Proceedings of the Society for Archaeological Sciences (Abstract) 1980. Strontium isotope characterization. [Google Scholar]
- 27.Capo R.C., Stewart B.W., Chadwick O.A. Strontium isotopes as tracers of ecosystem processes: theory and methods. Geoderma. 1998;82:197–225. [Google Scholar]
- 28.Van der Merwe N.J. Carbon isotopes, photosynthesis, and archaeology. Am. Sci. 1982;70:596–606. [Google Scholar]
- 29.DeNiro M.J., Schoeniger M.J. Stable carbon and nitrogen isotope ratios of bone collagen: Variations within individuals, between sexes, and within populations raised on monotonous diets. J. Archaeol. Sci. 1983;10:199–203. doi: 10.1016/0305-4403(83)90002-X. [DOI] [Google Scholar]
- 30.Lee-Thorp J.A., Sealy J.C., van der Merwe N.J. Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. J. Archaeol. Sci. 1989;16:585–599. doi: 10.1016/0305-4403(89)90024-1. [DOI] [Google Scholar]
- 31.Montgomery J. Lead and Strontium Isotope Compositions of Human Dental Tissues as an Indicator of Ancient Exposure and Population Dynamics. University of Bradford, United Kingdom; 2002. PhD thesis. [Google Scholar]
- 32.Nelson S.J., Ash M.M. 9th edition. Saunders Elsevier; 2010. Wheeler's Dental Anatomy, Physiology, and Occlusion. [Google Scholar]
- 33.Frost H.M. Skeletal structural adaptations to Mechanical Usage (SATMU): 1. Redefining Wolff’s Law: The bone modeling problem. Anat. Rec. 1990;226:403–413. doi: 10.1002/ar.1092260402. [DOI] [PubMed] [Google Scholar]
- 34.Pearson O.M., Lieberman D.E. The aging of Wolff’s “Law”: Ontogeny and Rresponses to mechanical loading in cortical bone. Am. J. Phys. Anthropol. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- 35.Teitelbaum S.L. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 36.Hill P.A. Bone remodelling. Br. J. Orthod. 1998;25:101–107. doi: 10.1093/ortho/25.2.101. [DOI] [PubMed] [Google Scholar]
- 37.Hedges R.E.M., Clement J.G., Thomas C.D.L., O'Connell T.C. Collagen turnover in the adult femoral mid-shaft: Modeled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 2007;133:808–816. doi: 10.1002/ajpa.20598. [DOI] [PubMed] [Google Scholar]
- 38.Jürimäe J. Interpretation and application of bone turnover markers in children and adolescents. Curr. Opin. Pediatr. 2010;22:494–500. doi: 10.1097/MOP.0b013e32833b0b9e. [DOI] [PubMed] [Google Scholar]
- 39.Tykot R.H. In: Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication and Evolution of Maize. Staller J., Tykot R., Benz B., editors. Academic Press; 2006. Isotope Analysis and the Histories of Maize; pp. 131–142. [Google Scholar]
- 40.Ambrose S., Norr L. In: Prehistoric human bone. Archaeology at the Molecular Level. Lambert J., Norr L., editors. Springer; 1993. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate; pp. 1–38. [Google Scholar]
- 41.Tieszen L.L., Fagre T. In: Prehistoric Human Bone, Archaeology at the Molecular Level. Lambert J., Grupe G., editors. Springer; 1993. Effect of diet quality and composition on the isotopic composition of respiratory CO2, bone collagen, bioapatite, and soft tissues; pp. 121–155. [Google Scholar]
- 42.Bird M.I., Crabtree S.A., Haig J., Ulm S., Wurster C.M. A global carbon and nitrogen isotope perspective on modern and ancient human diet. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2024642118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West J.B., Bowen G.J., Dawson T.E., Tu K.P. Springer-Verlag; 2010. Isoscapes. Understanding Movement, Pattern, and Process on Earth through Isotope Mapping. [Google Scholar]
- 44.Kootker L.M., Van Lanen R.J., Kars H., Davies G.R. Strontium isoscapes in the Netherlands. Spatial variations in 87Sr/86Sr as a proxy for palaeomobility. J. Archaeol. Sci.: Reports. 2016;6:1–13. doi: 10.1016/j.jasrep.2016.01.015. [DOI] [Google Scholar]
- 45.Willmes M., Bataille C.P., James H.F., Moffat I., McMorrow L., Kinsley L., Armstrong R.A., Eggins S., Grün R. Mapping of bioavailable strontium isotope ratios in France for archaeological provenance studies. Appl. Geochem. 2018;90:75–86. doi: 10.1016/j.apgeochem.2017.12.025. [DOI] [Google Scholar]
- 46.Snoeck C., Ryan S., Pouncett J., Pellegrini M., Claeys P., Wainwright A.N., Mattielli N., Lee-Thorp J.A., Schulting R.J. Towards a biologically available strontium isotope baseline for Ireland. Sci. Total Environ. 2020;712 doi: 10.1016/j.scitotenv.2019.136248. [DOI] [PubMed] [Google Scholar]
- 47.Lugli F., Cipriani A., Bruno L., Ronchetti F., Cavazzuti C., Benazzi S. A strontium isoscape of Italy for provenance studies. Chem. Geol. 2022;587 doi: 10.1016/j.chemgeo.2021.120624. [DOI] [Google Scholar]
- 48.Tipple B.J., Valenzuela L.O., Chau T.H., Hu L., Bataille C.P., Chesson L.A., Ehleringer J.R. Strontium isotope ratios of human hair from the United States: Patterns and aberrations. Rapid Commun. Mass Spectrom. 2019;33:461–472. doi: 10.1002/rcm.8378. [DOI] [PubMed] [Google Scholar]
- 49.Ammer S.T.M., Kootker L.M., Bartelink E.J., Anderson B.E., Cunha E., Davies G.R. Comparison of strontium isotope ratios in Mexican human hair and tap water as provenance indicators. Forensic Sci. Int. 2020;314 doi: 10.1016/j.forsciint.2020.110422. [DOI] [PubMed] [Google Scholar]
- 50.Shin W.-J., Gautam M.K., Shim J.-Y., Lee H.-S., Park S., Lee K.-S. Spatial distributions of strontium isotope ratios in human hair and tap water from South Korea. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.151352. [DOI] [PubMed] [Google Scholar]
- 51.INTERPOL Operation Indentify Me. 2023. https://www.interpol.int/How-we-work/Notices/Operation-Identify-Me
- 52.Kootker L.M., Plomp E., Ammer S. Tap water isotope data from the Netherlands. figshare. 2023 doi: 10.6084/m9.figshare.21946907.v1. [DOI] [Google Scholar]
- 53.Valenzuela L.O., Chesson L.A., O'Grady S.P., Cerling T.E., Ehleringer J.R. Spatial distributions of carbon, nitrogen and sulfur isotope ratios in human hair across the central United States. Rapid Commun. Mass Spectrom. 2011;25:861–868. doi: 10.1002/rcm.4934. [DOI] [PubMed] [Google Scholar]
- 54.Bender R.L., Dufour D.L., Valenzuela L.O., Cerling T.E., Sponheimer M., Reina J.C., Ehleringer J.R. Stable isotopes (carbon, nitrogen, sulfur), diet, and anthropometry in urban Colombian women: Investigating socioeconomic differences. Am. J. Hum. Biol. 2015;27:207–218. doi: 10.1002/ajhb.22640. [DOI] [PubMed] [Google Scholar]
- 55.Thompson A.H., Chesson L.A., Podlesak D.W., Bowen G.J., Cerling T.E., Ehleringer J.R. Stable isotope analysis of modern human hair collected from Asia (China, India, Mongolia, and Pakistan) Am. J. Phys. Anthropol. 2010;141:440–451. doi: 10.1002/ajpa.21162. [DOI] [PubMed] [Google Scholar]
- 56.Lehn C., Rossmann A., Graw M. Provenancing of unidentified corpses by stable isotope techniques – presentation of case studies. Sci. Justice. 2015;55:72–88. doi: 10.1016/j.scijus.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Hülsemann F., Lehn C., Schneider S., Jackson G., Hill S., Rossmann A., Scheid N., Dunn P.J.H., Flenker U., Schänzer W. Global spatial distributions of nitrogen and carbon stable isotope ratios of modern human hair. Rapid Commun. Mass Spectrom. 2015;29:2111–2121. doi: 10.1002/rcm.7370. [DOI] [PubMed] [Google Scholar]
- 58.Liversidge H.M. Technique and Application in Dental Anthropology. Cambridge University Press; 2008. Dental age revisited. [Google Scholar]
- 59.AlQahtani S.J., Hector M.P., Liversidge H.M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 2010;142:481–490. doi: 10.1002/ajpa.21258. [DOI] [PubMed] [Google Scholar]
- 60.Nudel I., Pokhojaev A., Bitterman Y., Shpack N., Fiorenza L., Benazzi S., Sarig R. Secondary Dentin Formation Mechanism: The Effect of Attrition. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18199961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coplen T.B. Normalization of oxygen and hydrogen isotope data. Chem. Geol. Isot. Geosci. 1988;72:293–297. [Google Scholar]
- 62.Chenery C.A., Pashley V., Lamb A.L., Sloane H.J., Evans J.A. The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite. Rapid Commun. Mass Spectrom. 2012;26:309–319. doi: 10.1002/rcm.5331. [DOI] [PubMed] [Google Scholar]
- 63.Daux V., Lécuyer C., Héran M.A., Amiot R., Simon L., Fourel F., Martineau F., Lynnerup N., Reychler H., Escarguel G. Oxygen isotope fractionation between human phosphate and water revisited. J. Hum. Evol. 2008;55:1138–1147. doi: 10.1016/j.jhevol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Tinoco R.L.R., Bastos M.Q.R., Machado C.E.P., Santos R.V., Rodrigues-Carvalho C. Isotopic analysis in teeth of contemporary brazilians with known diet and geolocation and its forensic value for human identification. Res. Soc. Dev. 2021;10 doi: 10.33448/rsd-v10i12.20243. [DOI] [Google Scholar]
- 65.Regan, L.A. (2006). Isotopic Determination of Region of Origin in Modern Peoples: Applications for Identification of U.S. War-Dead from the Vietnam Conflict. PhD thesis.
- 66.Juarez C.A. Strontium and Geolocation, the Pathway to Identification for Deceased Undocumented Mexican Border-Crossers: A Preliminary Report. J. Forensic Sci. 2008;53:46–49. doi: 10.1111/j.1556-4029.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 67.Åberg G., Fosse G., Stray H. Man, nutrition and mobility: A comparison of teeth and bone from the Medieval era and the present from Pb and Sr isotopes. Sci. Total Environ. 1998;224:109–119. doi: 10.1016/S0048-9697(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 68.Kamenov G.D., Curtis J.H. Using Carbon, Oxygen, Strontium, and Lead Isotopes in Modern Human Teeth for Forensic Investigations: A Critical Overview Based on Data from Bulgaria. J. Forensic Sci. 2017;62:1452–1459. doi: 10.1111/1556-4029.13462. [DOI] [PubMed] [Google Scholar]
- 69.O'Connell T.C., Hedges R.E.M., Healey M.A., Simpson A.H.R.W. Isotopic Comparison of Hair, Nail and Bone: Modern Analyses. J. Archaeol. Sci. 2001;28:1247–1255. doi: 10.1006/jasc.2001.0698. [DOI] [Google Scholar]
- 70.Ammer S.T.M., Bowen G.J., Davies G.R. Water Isotopes in Mexican Tap Water. Authorea. 2022 doi: 10.22541/au.166923551.15501678/v1. [DOI] [Google Scholar]
- 71.Saul T.B. University of Tennessee; 2017. A Exploration of the Effects of Taphonomy on Isotope Ratios of Human Hair.https://trace.tennessee.edu/utk_graddiss/4711 PhD dissertation. [Google Scholar]
- 72.Gordon G., Saul T., Steadman D., Wescott D., Knudson K., Anbar A. U.S. Department of Justice report 2014-DN-BX-K002. 2018. The Isotopic Taphonomy of Human Remains. [Google Scholar]
- 73.Plomp E., von Holstein I.C.C., Koornneef J.M., Smeets R.J., Baart J.A., Forouzanfar T., Davies G.R. Evaluation of neodymium isotope analysis of human dental enamel as a provenance indicator using 1013 Ω amplifiers (TIMS) Sci. Justice. 2019;59:322–331. doi: 10.1016/j.scijus.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kootker L.M., Plomp E., Font L., Ammer S.T.M. Tooth Isotope Data from the Netherlands. Figshare. 2023. [DOI]
- 75.Ammer S.T.M., Kootker L.M. Human Hair Isotope Data from the Netherlands. Figshare. 2023. [DOI]
- 76.Fischer E., Saller K. Eine neue Haarfarbentafel. Anthropol. Anzeiger. 1928;5:238–244. [Google Scholar]
- 77.Stanborough R.J. How to Identify and Style Your Hair Type. Healthline. 2019 https://www.healthline.com/health/beauty-skin-care/types-of-hair [Google Scholar]
- 78.Krause K., Foitzik K. Biology of the Hair Follicle: The Basics. Semin. Cutan. Med. Surg. 2006;25:2–10. doi: 10.1016/j.sder.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Kootker L.M., Mbeki L., Morris A.G., Kars H., Davies G.R. Dynamics of Indian Ocean slavery revealed through Isotopic data from the Colonial era Cobern Street burial site, Cape Town, South Africa (1750-1827) PLoS One. 2016;11 doi: 10.1371/journal.pone.0157750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irrgeher J., Galler P., Prohaska T. 87Sr/86Sr isotope ratio measurements by laser ablation multicollector inductively coupled plasma mass spectrometry: Reconsidering matrix interferences in bioapatites and biogenic carbonates. Spectrochim. Acta B Atom Spectrosc. 2016;125:31–42. [Google Scholar]
- 81.Linderholm A., Kılınç G.M., Szczepanek A., Włodarczak P., Jarosz P., Belka Z., Dopieralska J., Werens K., Górski J., Mazurek M., et al. Corded Ware cultural complexity uncovered using genomic and isotopic analysis from south-eastern Poland. Sci. Rep. 2020;10:6885. doi: 10.1038/s41598-020-63138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Klinken G.J. Bone Collagen Quality Indicators for Palaeodietary and Radiocarbon Measurements. J. Archaeol. Sci. 1999;26:687–695. doi: 10.1006/jasc.1998.0385. [DOI] [Google Scholar]
- 83.Ambrose S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990;17:431–451. doi: 10.1016/0305-4403(90)90007-R. [DOI] [Google Scholar]
- 84.DeNiro M.J. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature. 1985;317:806–809. [Google Scholar]
- 85.Brock F., Wood R., Higham T.F.G., Ditchfield P., Bayliss A., Ramsey C.B. Reliability of nitrogen content (%N) and carbon:nitrogen atomic ratios (C:N) as indicators of collagen preservation suitable for radiocarbon dating. Radiocarbon. 2012;54:879–886. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
De-identified human hair and tooth isotope data are deposited on Figshare and publicly available as of the date of publication.74,75
-
•
Tap water isotope data supporting the findings of this study are deposited on Figshare and publicly available as of the date of publication.52
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.