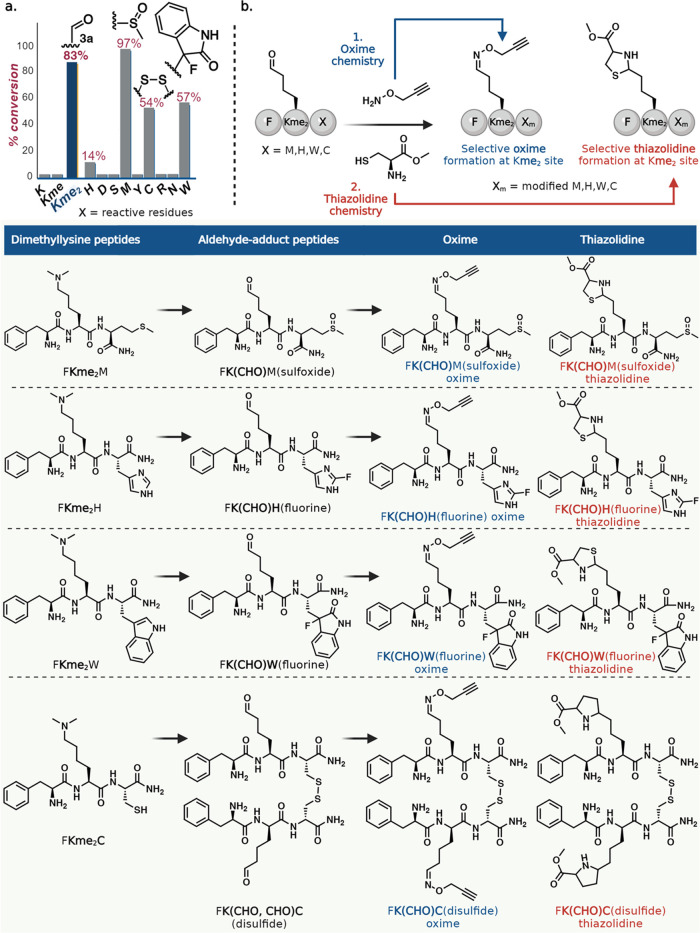
Figure 3.
Evaluation of chemoselectivity of the TACO reaction. (a) Chemoselectivity studies of the TACO reaction with varying tripeptides FXV, where X = K, Kme, Kme2, H, D, S, M, Y, C, R, N, and W. Only the Kme2 residue modified to an aldehyde under the reaction conditions. Side adducts were observed with histidine (fluorination), methionine (sulfoxide), tryptophan (fluorination and oxygenation), and cysteine (disulfide). (b) Selective derivatization of Kme2-generated aldehydes on tripeptides FKme2X, where X = M, H, W, and C, in the presence of side adducts on histidine, methionine, cysteine, and tryptophan using oxime and thiazolidine chemistry in NaP buffer. The presence of TACO-generated adducts did not interfere with subsequent modification of dimethyl lysine sites, thus indicating the negligible effect of the adducts on downstream application of TACO for profiling of lysine dimethylome.