Figure 5.
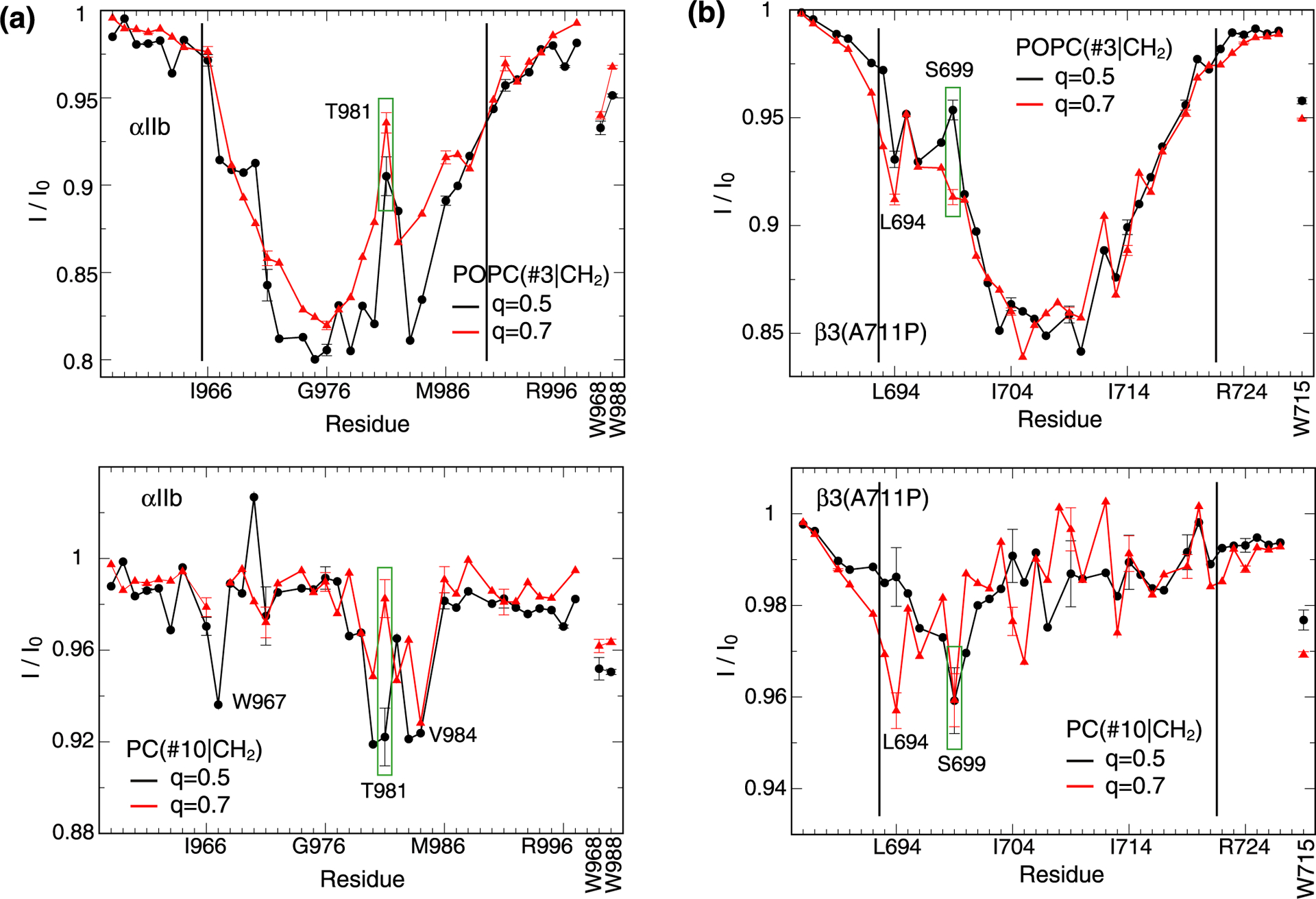
Influence of bicelle size (q-factor) on protein-lipid contacts. Saturation transfers between protein backbone HN nuclei of (a) integrin αIIb and (b) β3(A711P) TM segments and either POPC(#3|CH2) or PC(#10|CH2) nuclei in q=0.5 and 0.7 bicelles. Tryptophan sidechain HN nuclei were also evaluated as shown using vertical labels. Black vertical lines indicate the previously determined membrane borders of αIIb and β3 TM helices.17,18 The POPC(#3|CH2)-HN transfer at the TM helix center of αIIb at q=0.7 is less efficient than at q=0.5 suggesting a heightened proportion of model II (Figure 2c) in q=0.7 bicelles.
