Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the second most common cause of cancer death worldwide. Current treatment options for patients with intermediate and advanced HCC are limited, and there is an unmet need for novel therapeutic approaches. HCC is an attractive target for immunomodulation therapy, since it arises in an inflammatory milieu due to hepatitis B and C infections and cirrhosis. However, a major barrier to the development and success of immunotherapy in patients with HCC is the liver’s inherent immunosuppressive function. Recent advances in the field of cancer immunology allowed further characterization of immune cell subsets and function, and created new opportunities for therapeutic modulation of the immune system. In this review, we present the different immune cell subsets involved in potential immune modulation of HCC, discuss their function and clinical relevance, review the variety of immune therapeutic agents currently under investigation in clinical trials, and outline future research directions.
Keywords: Hepatocellular carcinoma, HCC; Immunotherapy; Checkpoint blockade; Cancer vaccines; Review article; Cancer immunology
Introduction
Hepatocellular carcinoma (HCC) is the most common liver malignancy, constituting about 80% of primary liver tumors [1]. The incidence of HCC is rapidly rising in Japan, Europe, and North America due to increased incidence of HCV infection and non-alcoholic fatty liver disease, and in Africa and Middle East due to HBV infection [2, 3].
Accepted options for treatment of the early stage HCC include liver resection, liver transplantation, and ablation (radiofrequency or microwave). Patients with intermediate stage disease are selected for locoregional therapy including trans-arterial chemoembolization (TACE) and radioembolization. These patients have relatively high 5-year survival rates of up to 70% compared to a dismal 16% in patients with advanced stage disease [4]. The only available treatment for the latter group is sorafenib, a tyrosine-kinase inhibitor that has a limited survival benefit of 3 months [5]. Accordingly, a novel therapeutic approach is desperately needed.
HCC is a uniquely immunosuppressive cancer
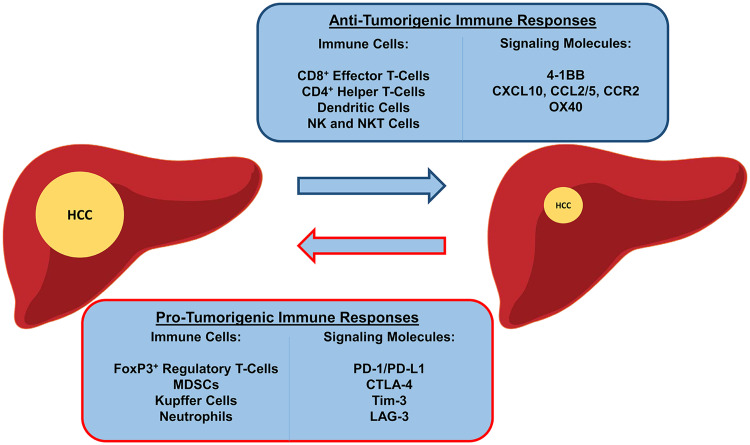
There is a growing evidence to suggest that HCC may be considered an immunogenic tumor, arising in an immunosuppressive environment. The chronic inflammation, viral etiology, and cirrhosis underlying the formation of most HCC tumors highlight an intricate relationship between the immune biology and the development of this neoplasm [6]. The liver is constitutively immunosuppressive [6], as it promotes systemic tolerance to foreign antigens [7], which prevents excessive reactions to toxins and antigens draining from the enteric circulation [8]. The HCC tumor microenvironment (TME) has immunosuppressive features due to the chronic nature of the disease and to tolerogenic liver properties that include a combination of:
Active T regulatory cell (T-reg) compartment [9] that will be amply discussed in this work.
Underlying T-cell exhaustion due to the chronic inflammation of underlying chronic liver disease [10].
Abundance of inhibitory myeloid cells or myeloid-derived suppressor cells (MDSC) due to their accumulation in the liver [11]. MDSCs are a heterogeneous population of immature myeloid cells [12, 13] that suppress T-cell responses through the depletion of l-arginine [14] and l-cysteine [15], release of reactive oxygen and nitrogen species [16], and the induction of T-regs [17]. They also inhibit NK-cell cytotoxicity and cytokine secretion [12]. A subset of MDSC (CD14+HLA-DR−/low) was found to inhibit immunity in HCC by inducing T-regs [17]. Increased MDSCs are reported as a negative prognostic indicator when found in the pre-treatment peripheral blood of patients receiving hepatic arterial infusion chemotherapy [18] and post-treatment in patients receiving radiation therapy [19]. Accordingly, MDSCs play a suppressive role in the development of HCC tumor immunity.
Liver resident macrophages or Kupffer cells (KC) make up about 80% of the macrophages in the body [20]. During homeostasis, they maintain immune tolerance through anti-inflammatory functions [21]. KCs can secrete IDO [22] and IL-10 [8, 23] which suppress local immunity. In chemically induced murine HCC, KCs were found to promote carcinogenesis [24], and in HCC patient studies, KCs were found to inhibit anti-tumor CD8+ T-cell killing through PD-L1 signaling [25, 26]. Therefore, KCs are pro-tumorigenic cells central to the liver’s unique immunosuppressive function.
The immunosuppressive enzyme arginase 1 is commonly expressed by HCC tumor cells [27]. Immunohistochemical detection of arginase 1 in unknown metastases is used to identify an HCC primary source [28].
HCC exploits this immune tolerance to initiate and promote HCC carcinogenesis and progression. These characteristics of HCC may steer immunotherapeutic strategies to those that inhibit immune suppressive mechanisms, rather than directly increase immune effector function.
Biology of immune cells and molecules used in immunotherapy for HCC
T-regs are immune inhibitory cells that occur naturally and function mainly in self-tolerance and prevention of autoimmunity [29]. They are a subset of CD4+ T-cells that are FoxP3+ [30], and generally express the interleukin 2 receptor CD25 [31, 32] and the activation markers OX40 and CD69 [33]. More recently, three subtypes of CD4+FoxP3+ cells are characterized: a resting phenotype (FoxP3lowCD45RA+CD25low), an inhibitory phenotype (FoxP3hiCD45RA−CD25hi), and a non-T-reg pro-inflammatory phenotype (FoxP3lowCD45RA−CD25 low) [34]. T-regs reduce CD8 killer T-lymphocyte function by inhibiting their proliferation, activation, and degranulation [35], through secretion of IL-10 and TGF- β and the action of CTLA4 and CD39/CD73 [33, 36–39]. They also decrease NK-cell cytotoxic activity and IFNγ production [40]. T-regs are found in higher concentrations in HCC tumors and blood of HCC patients compared to those of healthy controls [9, 41]. T-regs are also thought to increase the chronicity of HCV and HBV infections promoting progression to HCC [42, 43]. Increased FoxP3+ infiltration in HCC specimens is associated with worse patient survival [30, 35, 44, 45] and higher recurrence rates [46]. HCC induces T-reg formation and potentiates their effect by secreting TGF-β. A higher TGF-β expression in tumor cells is reported to be a marker of worse patient outcomes [47]. CD8+FoxP3+ T-regs were characterized in HCC and were found to be associated with more advanced tumor stage [48], suggesting a role in tumor progression. Inhibiting T-reg functions in HCC may be valuable addition to other immunotherapeutic strategies.
CD8 + effector T-cells have been repeatedly associated with improved prognosis in several malignancies including breast, lung, colon cancers, and melanoma [49–52]. However, clinical data describing the effects of CD8 tumor infiltration on HCC patient outcomes are conflicting. A recent large study conducted in 499 patients with HCC, found CD8 tumor infiltration to be associated with better patient overall (OS) and disease-free survival (DFS) [53, 54]. The spatial distribution of CD8+ TILs may influence their efficiency in tumor clearance, since their presence in the center of the tumor rather than its margin correlates with more favorable outcomes [53]. Thus, there is a basis for hypothesizing that T-cells recognizing specific HCC antigens may have a role in controlling HCC. Supporting this notion, T-cells were found to react to specific tumor antigens such as glipican-3, NY-ESO-1, hTERT, and p53 in patients with HCC [55–58]. On the other hand, it has been reported that CD8 infiltration in the center of tumors < 3 cm in diameter can be indicative of higher recurrence rates in HBV-associated HCC patients [59]. Several other studies did not identify a correlation between CD8+ TILs and clinical outcomes in HCC [30, 45, 60, 61]. This could be due to the role that other immune cells may play in generating an immune response. In a cohort of 302 HCC patients, longer patient survival was observed in the group of patients with high activated CD8+ TILs/T-reg ratio but not with high CD8+ TIL alone [30]. There is no clear consensus on the prognostic significance of CD8+ T-cell density in HCC tissue; this calls for a better characterization of the role of cytotoxic T-lymphocytes in HCC.
CD4 + T-cells are essential for the establishment of an effective anti-tumor response. They include multiple subtypes that balance the activation of immune cells. Most prominently affecting CD8+ T-cell upregulation and downregulation are the T helper 1 cells and the FoxP3+ T-reg cells, respectively [62]. A recent experimental model described an association between the loss of CD4+ T-cells and HCC development in mice with non-alcoholic fatty liver disease; this association was further validated in human HCC samples [63]. In another study, increasing numbers of CD4+ T-cells expressing granzyme A, B and perforin called CD4+ cytotoxic T-lymphocytes was found to have a positive effect on patient outcomes [64]. The available data indicate an anti-tumorigenic role of CD4+ cytotoxic T-lymphocytes in HCC.
DCs are pivotal antigen-presenting cells in the initiation of host defense against immune insults. They prime the adaptive immune response against HCC cells [65]. Mouse models suggest that tumor antigen pulsed DC vaccines decrease HCC size and increase survival through the activation of natural killer T (NKT) cells and CD8+ and CD4+ T-cells [66]. DC vaccines also potentiate a reduction in immune suppressive T-regulatory cells and tumor growth factor secretion in the TME [67].
NK cells are part of innate immunity, yet they share some features with adaptive immune cells [68]. NK cells can recognize specific ligands on tumor cells such as MHC class I related chains A and B, and support the development of adaptive immunity [68–70]. Their physiological localization in liver sinusoids predisposes them to be more prevalent in liver tumors including HCC than in other cancers. Hepatic NK cells tend to be more cytotoxic than the hematogenous ones and may have a role in immune modulated response to metastasizing tumor cells [71]. Furthermore, the decreased expression of CD155, a modulator of NK-cell function, was found to be associated with worse HCC patient outcomes [72, 73]. NK cells may have a role in the defense against HCC tumors.
Natural killer T (NKT) cells express receptors common to T-cells including CD4 and CD3 [74]. Enhanced reactivity of NKT cells (as identified by combinations of CD8 and NK1.1 markers or CD3 and DX-5 markers) to tumor antigens proved to be effective in suppressing HCC [66, 75]. Activated NKT cells, or cytokine induced killers (CIK), have been investigated in the treatment of HCC patients as will be discussed later.
PD-1 and its ligand PD-L1 constitute an immune regulating checkpoint with a well-established role in cancer progression. Their role in HCC progression is currently being characterized based on their involvement in the development of chronic HBV [76] and HCV [77] infections. PD-L1 expression on HCC tumor cells is a marker of shortened patient DFS [78] and OS [79, 80]. HCC cells tend to minimally express PD-L1 [25] which regulates the interaction between hepatic macrophages and CD8+ PD-1+ T cells [25, 26]. Provocative results from ongoing clinical trials testing the efficacy of PD-1/PD-L1 blocking antibodies in HCC are presented further along this article.
CTLA4 inhibits T-cell function by competing with an activating surface molecule (CD28) for the binding of CD80 and CD86 on antigen-presenting cells [81]. CTLA4 is also essential for the production of the immune suppressors IL-10 and IDO when expressed on DC in the HCC TME [82]. CTLA4 is active in the HCC TME, making it a popular target for cancer treatment.
Lymphocyte activation gene (LAG-3) is a CD4-like molecule [83] expressed on activated T and NK cells [84]. LAG-3 binds to MHC-II or Galectin-3 and negatively regulates T-cell function [85, 86]. It is an attractive immunotherapeutic target in many cancers alone or in combination [87], especially that LAG-3 does not compete with CD4 for MHC-II binding, and, therefore, does not affect CD4 T-cell-mediated effector functions [88]. In HCC, increased LAG-3 expression correlates with a decrease in the activity of anti-HBV-specific CD8+ T-cells [89]. LAG-3 is currently being investigated as a single target or in combination with anti-PD-1 and PD-L1 in HCC patients.
TGF-β signaling regulates cell differentiation, proliferation, motility, death [90], and angiogenesis [91]. It plays a potent immunosuppressive role in HCC by inhibiting T- and NK-cell activation [92]. Furthermore, it has a central role in epithelial–mesenchymal transition that contributes to HCC metastasis [93, 94]. Increased TGF-β expression in HCC tumor cells [95] and patient serum [96] are associated with worse patient outcomes. Targeting TGF-β signaling is an attractive strategy for treatment of HCC patients and is currently in clinical trials as discussed later.
A summary of the immune landscape in HCC is illustrated in Fig. 1.
Fig. 1.
Immune landscape in HCC. The immune cell subsets involved in tumor promotion and tumor suppression. CCL chemokine ligand, CCR chemokine receptor, CXCL chemokine C-X-C motif ligand, LAG-3 lymphocyte activation gene, OX40 CD134, Tim-3 T-cell immunoglobulin mucin 3
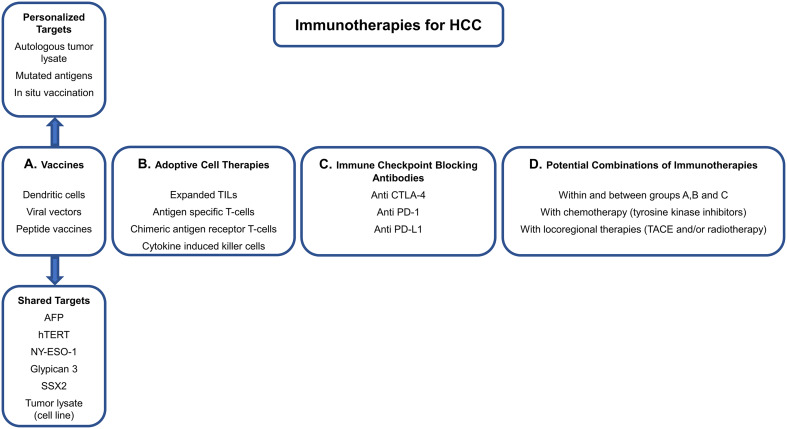
Immunotherapy in HCC management (Fig. 2)
Fig. 2.
Current immunotherapeutic strategies. Immunotherapeutic opportunities available for current and future clinical trials, and possible combinations. TACE transarterial chemoembolization
The unique immunosuppressive HCC tumor microenvironment described above makes it an attractive target for immunotherapy, particularly immune checkpoint inhibitors. In this section, we present ongoing clinical trials and currently available results.
Cancer Vaccines are conceptually attractive for cancer therapy due to their limited adverse effect profile and potential for antigen-specific anti-tumor effects [97]. Prophylactic vaccines such as the HBV vaccine prevent viral infection and development of chronic liver disease, thus, dramatically decreasing the risk of developing HCC [98]. Therapeutic cancer vaccines are designed to target TAA which are specific for tumor cells or overexpressed on cancer cells. HBV and HCV antigens are unique to the HCC malignancy and present attractive therapeutic targets [99]. Several vaccination strategies are used to target cancer cells including tumor lysate that contains all TAAs and peptide vaccines that contain specific TAAs or full-length proteins. Those targets can also be pulsed on dendritic cells and some can be encoded in DNA or viral vectors (Table 1). These vaccines prime the immune system to target TAAs presented by MHC-I or MHC-II molecules in the TME and tumor draining lymph nodes. Among the most extensively explored cancer vaccine targets in HCC are: carcinoembryonic antigen, NY-ESO-1, alpha-fetoprotein, glypican-3 (GPC-3), melanoma-associated antigen, and human TERT [100]. While the majority of cancer vaccines have demonstrated enhanced immune responses against the targets, clinical efficacy has been limited [101] (Table 1). Glypican-3, the most studied target for HCC therapy, is overexpressed in 80% of HCC tumors [102]. GPC-3 expression in tumor cells is associated with decreased patient survival [103]. In clinical trials, a peptide vaccine targeting GPC-3 led to disease control [partial response (PR) and stable disease (SD)] for 2 months in 61% of patients (3% PR and 58% SD of which 21% had responses that do not meet criteria for PR), was well tolerated, and patients with higher anti GPC-3-specific lymphocyte frequencies had better OS [104]. In a follow-up phase II single arm trial, this vaccine resulted in a trend toward decreased recurrence rates after surgery in patients with GPC-3 positive tumors compared to selected case–control patients (24% compared to 43% with surgery alone, p = 0.054, Table 1) [105] with minimal side effects.
Table 1.
Cancer vaccine clinical trials for HCC patients
| Trial Identifier | Phase | Vaccine | Route | Target | Patient population | N | Status | Results |
|---|---|---|---|---|---|---|---|---|
| UMIN-CTR# 000001395 | I | Peptide | Intradermal | GPC-3 | Advanced disease, HLA-A2 or A24 | 33 | Complete | PR (3%), SD (58%) [104] |
| UMIN-CTR# 000002614 | II | Peptide | Intradermal | GPC-3 | Post resection and RFA | 41 | Complete | Improved 1 year RFS vs surgery only [105] |
| NCT01522820 | I | Fusion protein (with DEC-205)/sirolimus | Intranodal | NY-ESO-1 | Post resection and TACE, NY-ESO-1 expressing tumors | 30 | Ongoing | NA |
| NCT02133079 | I/II | Autologous tumor derived protein | NA | Heat Shock Protein gp96 | Post resection | 20 | Ongoing | NA |
| NCT02232490 | III | HBV-HCV derived antigens | NA | Hepcortespenlisimut-L | Advanced disease | 100 | Ongoing | NA |
| NCT02256514 | II | HBV-HCV derived antigens | Oral | Hepcortespenlisimut-L | Advanced disease | 120 | Ongoing | NA |
| NCT02338778 | I/II | DPT, staphylococcus aureus, typhoid and paratyphoid | Intravenous | Multiple vaccines as adjuvants | All | 20 | Complete | NA |
| NCT01974661 | I | DC | Intratumoral | All TAAs | After TACE ± sorafenib | 18 | Ongoing | NA |
| NCT01828762 | I | DC | Subcutaneous | Irradiated tumor cells | 1ary HCC after TACE | 8 | Complete | NA |
| NCT00004604 | I | DC | Intravenous | CEA | All CEA expressing solid tumors | 24 | Complete | NA |
| NA | II | DC | Intravenous | HepG2 cell line | Advanced disease | 35 | Complete | PR (4%), SD (24%) [106] |
| NCT00629759 | I | Oncolytic virus JX-549 + Sorafenib | Intratumoral | All TAAs | Non-resectable | 14 | Complete | Well tolerated, decreased tumor perfusion and necrosis [137] |
| NCT02509507 | I | T-VEC | Intratumoral | All TAAs | Advanced HCC and liver metastases | 100 | Ongoing | NA |
CEA carcinoembryonic antigen; DPT diphtheria, pertussis, tetanus; NA not available; RFA radiofrequency ablation; RFS recurrence free survival; T-VEC talimogene laherparepvec
Patient-derived DCs present another popular method of cancer vaccine delivery. This treatment consists of isolating patient’s DCs and pulsing them with a specific antigen or tumor lysate then re-infusing them. Multiple studies used this vaccination approach to target a variety of antigens. In HCC patients, one trial tested the efficiency of DCs pulsed with tumor lysate in 35 patients with unresectable disease. Most patients had increased IFN-γ and decreased AFP levels following vaccination, suggesting immunological response. Disease control rate according to the International Union Against Cancer Criteria for ≥ 3 months was 28% with no significant toxicities observed (PR in 1 patient and SD in 6) [106]. Targeting tumor antigens using peptide vaccines administered directly or pulsed on DCs is an attractive modality for the treatment of HCC that should be combined with immune checkpoint inhibitors in clinical trials.
Intratumoral in situ vaccination using oncolytic virus with or without immune adjuvant is another approach that leads to the release of tumor antigens. A phase 2 study of the intratumoral vaccinia virus (JX-549) in 30 patients with unresectable HCC showed a 50% disease control rate at 8 weeks and median survival of 14.1 month and resulted in a single-grade 4 toxicity. A GM-CSF producing oncolytic herpes simplex virus (T-VEC) was approved for treatment of unresectable stage IIIB/C and IV melanoma in October 2015. T-VEC is currently in a phase I study for advanced HCC (NCT02509507). There may be promise for extending the benefits of this approach to HCC, alone or in combination with other modalities.
-
(b)
Adoptive cell therapy is a novel immunotherapy method where patient-derived T or NKT cells are expanded and activated ex vivo then re-infused. Based on the source and the method used for cell activation, adoptive cell transfer could be classified into: (1) TILs, (2) engineered T-cells that are specific for cancer antigens, (3) T-cells that express a chimeric antigen receptor consisting of antibody bound to the T-cell receptor’s intracellular domain, and (4) CIKs consisting of CD3+ CD56+ NKT cells activated with a cocktail of anti-CD3 antibodies, IL-2, IL-1α, and IFNγ [107]. Treatment with T-cells specific to AFP is showing promising results in pre-clinical studies and a clinical trial using this approach is currently ongoing [108]. Furthermore, a retrospective study of over 400 case/control HCC patients receiving surgery and CIK or surgery alone showed a significant survival benefit of CIK administration in multivariate analysis [109]. The same group showed, in a randomized controlled study including 200 patients, that CIK treatment significantly prolonged median time to recurrence, but did not significantly prolong OS or DFS [107] compared to standard treatment. In Korea, another randomized controlled trial included 230 patients with the early stage HCC post-complete resection. Patients who received CIK post-op had significantly lower hazard ratio of any death and of cancer-related deaths [110]. Similar results were also found in a systematic review that included 13 randomized phase II and III studies [111]. The adoptive cell transfer therapy is currently under investigation in many solid tumors including HCC and the results of these studies are eagerly awaited (Table 2).
-
(c)
Immune checkpoint blockade since 2014, the FDA has approved checkpoint blocking antibodies for patients with melanoma, lung, head and neck, bladder and renal cancers, Hodgkin lymphoma, and multiple myeloma. The indication of immune checkpoint inhibitors is likely to extend beyond these indications. Many clinical trials are currently testing immune checkpoint blockade in HCC (Table 3). The CTLA4 checkpoint inhibitor tremelimumab was studied in 20 HCC patients in the setting of chronic HCV cirrhosis and mostly Child–Pugh class B, at a dose of 15 mg/kg every 90 days [112]. Disease control was achieved in 76% (18% PR, 59% SD, according to RECIST criteria) of the 17 patients assessed for response at 3 months, along with an observed decrease in HCV viral load in most patients. Importantly, no severe immune-mediated adverse events occurred, and steroid rescue was not required. The PD-1 inhibitor nivolumab has been studied in 262 HCC patients in the non-comparative phase 1/2 CheckMate-040 trial. Disease control was achieved in 64% of the 214 patients treated with nivolumab at 3 mg/kg (1% complete response, 18% PR, 45% SD, according to RECIST criteria); follow-up is currently ongoing. Nivolumab demonstrated limited antiviral activity; responses were independent of previous sorafenib treatment, HBV or HCV infection, and tumor cell PD-L1 expression. PD-L1 was expressed on ≥ 1% of tumor cells in 20% of assessed tumors and in 26% (95% confidence interval: 13–44%) of tumors with objective response [113]. A subsequent randomized phase of this study comparing nivolumab to sorafenib is ongoing. CheckMate-459 trial is also ongoing [114]; it is a phase III randomized, multi-centered trial of nivolumab vs sorafenib in patients with Child–Pugh Class A cirrhosis in 726 patients (NCT02576509, Table 3). Another PD-1 blocking therapy, pembrolizumab, is currently being investigated in the second-line setting in HCC (NCT02702401). Biomarkers of immune response to immune checkpoint blockade including PD-L1 expression on tumor cells, lymphocytic infiltration, and mutational load warrant thorough testing in the HCC setting. These biomarkers have the potential to narrow patient selection and, therefore, increase response rates. The outcome of immune checkpoint inhibitors in HCC is promising and is likely to gain more traction as the final results of these studies are revealed.
-
(d)
TGF-β receptor inhibitor a novel immunotherapeutic modality constituted of a small molecule galunisertib (LY2157299) which inhibits TGF-β receptor signaling is being investigated in the treatment of HCC patients. As discussed earlier, TGF-β signaling has immunological role in promoting HCC progression. Interim analysis from 109 HCC patients who progressed on sorafenib and treated with galunisertib showed decrease in AFP levels by > 20% after treatment in 24% of patients. Patients who had a decrease in AFP had a longer median OS (21 vs 7 months, p = 0.0006) [115].
-
(e)
Combination therapies several locoregional therapies have been shown to increase antigen presentation and elicit significant immune responses in HCC. Increased infiltration of dendritic cells and activated T-cells has been demonstrated in patients treated with radiofrequency ablation [116, 117]. Similar immune stimulation has been documented in patients treated with TACE [54]. These ablative and locoregional therapies may function as autologous cancer vaccines by abruptly exposing cancer antigens to the immune system. In some instances, shrinkage of distant untreated tumors (abscopal effect) was observed [118, 119]. A recent study of HCC patients (Child–Pugh class A/B7 cirrhosis) treated with tremelimumab followed by TACE or radiofrequency ablation showed a 23% partial response rate in patients evaluable for response outside of locally treated lesions [120]. While no complete responses were reported, there was a tolerable adverse event profile, with pruritus occurring most commonly. Interestingly, responders had increased CD4/T-reg and CD8/T-reg ratios and a marked decrease in HCV viral load.
Table 2.
Adoptive cell transfer clinical trials for HCC patients
| Trial identifier | Phase | Cell type | Target | Patient population | N | Status | Results |
|---|---|---|---|---|---|---|---|
| NCT00699816 | III | T-cells | All TAAs | Stage I or II after curative resection | 230 | Complete | NA |
| NCT00769106 | III | CIK | All TAAs | After radical resection | 200 | Complete | Prolonged median time to recurrence [107] |
| NA | III | CIK | None | After radical resection | 230 | Complete | Prolonged OS and CSS [110] |
| NCT01147380 | I | NK cells, IL-2 stimulated | All TAAs | After liver transplant | 18 | Complete | 0% adverse events |
| NCT01462903 | I | TILs, IL-2 stimulated | All TAAs | Metastatic | 20 | Ongoing | NA |
| NCT01758679 | IV | CIK | I-131 | After resection | 120 | Ongoing | NA |
| NCT01801852 | I | NKT cells | All TAAs | Metastatic | 300 | Ongoing | NA |
| NCT01821482 | II | DC and CIK | All TAAs | After resection or TACE | 100 | Ongoing | NA |
| NCT01897610 | II | T-cells and/or Sorafenib | All TAAs | Stage III or IV | 40 | Ongoing | NA |
| NCT02008929 | II | NK | All TAAs | After resection | 5 | Ongoing | NA |
| NCT02026362 | I/II | DC and T-cells | 17 total TAAs | After resection or RFA | 100 | Ongoing | NA |
| NCT02425735 | I/II | DC-CIK and/or T cell | I-131 | After resection | 40 | Complete | NA |
| NCT02487017 | II | DC and/or CIK | All TAAs | After TACE | 60 | Ongoing | NA |
| NCT02568748 | III | CIK | All TAAs | After TACE | 20 | Ongoing | NA |
| NCT02632006 | I/II | T cell | PD-1 | Advanced | 40 | Ongoing | NA |
| NCT02632188 | I/II | T cell | Multiple TAAs | After resection | 60 | Ongoing | NA |
| NCT02638857 | I/II | T cell | Multiple TAAs | After TACE | 60 | Ongoing | NA |
| NCT02662348 | I | T cell | CD3 + HER-2 | All, HER-2 expressing tumors | 6 | Ongoing | NA |
| NCT02678013 | III | T cell | All TAAs | After RFA | 210 | Ongoing | NA |
| NCT02709070 | III | T cell | All TAAs | After resection | 210 | Ongoing | NA |
| NCT02715362 | I/II | T cell | GPC-3 | Unresectable GPC-3 expressing HCC | 30 | Ongoing | NA |
| NCT02723942 | I/II | T cell | GPC-3 | Advanced GPC-3 expressing HCC | 60 | Ongoing | NA |
CSS cancer-specific survival, NA not available
Table 3.
Immune checkpoint inhibitors and combination therapy clinical trials for HCC patients
| Trial identifier | Phase | Target | Drug | Other treatments | Patient population | N | Status | Results |
|---|---|---|---|---|---|---|---|---|
| NCT01008358 | II | CTLA4 | Tremelimumab | None | Chronic HCV + unresectable | 20 | Completed | NA |
| NCT02595866 | I | PD-1 | Pembrolizumab | None | Unresectable | 39 | Ongoing | NA |
| NCT01853618 | I | CTLA4 | Tremelimumab | TACE or RFA | Advanced | 100 | Ongoing | PR: 23% [120] |
| NCT02239900 | I/II | CTLA4 | Ipilimumab | SBRT | Unresectable | 120 | Ongoing | NA |
| NCT02576509 | III | PD-1 | Nivolumab | Sorafenib | Advanced | 726 | Ongoing | NA |
| NCT02702401 | III | PD-1 | Pembrolizumab | Best supportive care | Resistant to sorafenib | 408 | Ongoing | NA |
| NCT01658878 | I |
PD-1 CTLA4 |
Nivolumab and/or ipilimumab | Sorafenib | Advanced | 91 | Ongoing | ORR: 9%. And 6mo OS rate: 69% [138] |
| NCT02821754 | I/II |
CTLA4 PD-L1 |
Durvalumab and/or tremelimumab | TACE or RFA | Resistant to sorafenib and chemotherapy | 90 | Ongoing | NA |
| NCT02519348 | I/II |
CTLA4 PD-L1 |
Tremelimumab and/or durvalumab | None | Unresectable | 144 | Ongoing | NA |
| NCT02572687 | I |
PD-L1 VEGF |
Ramucirumab and durvalumab |
None | Resistant to sorafenib | 114 | Ongoing | NA |
| NCT02795429 | I/II |
cMET PD-1 |
Capmatinib and/or PDR001 | None | Advanced | 108 | Ongoing | NA |
| NCT02562755 | III | Oncolytic virus | Pexa Vec and sorafenib | Sorafenib | Advanced | 600 | Ongoing | NA |
cMET tyrosine-protein kinase MET, ORR overall response rate, RFA radiofrequency ablation, SBRT stereotactic body radiation therapy
A recent study combined cyclophosphamide which suppresses T-reg activity [121] with low-dose hepatic radiation (3.5 Gy over 3 days) and adjuvant intratumoral injection of poly-ICLC (a Toll-like receptor-3 agonist) along with arterial embolization [122]. This study included 25 liver cancer patients and showed a mean survival of 26 months, two patients were down-staged and proceeded to transplantation, and one patient was alive at 87 months. This locoregional therapy modality may also be used in the future for tumor immune-embolization by locally injecting antibodies against specific immune targets with the goal of inducing a localized and systemic immune response.
Tyrosine-kinase inhibitors such as sorafenib have immunomodulatory effects including reducing T-regs [123] and inhibiting MDSC [124]. Sorafenib is currently investigated in combination with anti-PD-1 (PDR001) in HCC. The result of this study may reveal a synergistic effect of these two agents; however, the toxicity profile of combining these two modalities needs to be clearly determined.
Many immune checkpoint inhibitors are currently investigated in combination in HCC based on the promising outcomes of single agents. Anti-CTLA4 (tremelimumab) and anti-PD-L1 (durvalumab) are currently being evaluated in combination with TACE or radiofrequency ablation and compared to single immune checkpoint inhibitor in patients with unresectable HCC with or without HBV or HCV who progressed on sorafenib [125]. Combination of anti-CTLA4 (ipilimumab) and anti-PD-1 (nivolumab) is also currently being investigated in clinical trials (Table 3). The orally administered anti-TGF-β (galunisertib) in combination with nivolumab are ongoing supported by pre-clinical data that showed silencing the TGF-β pathway markedly increases sensitivity to anti-CTLA4 and anti-PD-1 antibodies [126] (Table 3). The role for combination therapies in HCC remains to be determined. These combinations should be investigated based on solid rationale for synergy and should be compared to single checkpoint blockade to determine the relative risk/benefit of additional treatment.
Immune targets not yet explored in HCC patients
4-1BB (CD137) is a member of the TNF receptor superfamily. It is expressed on T- and NK-cell membranes, where its ligation inhibits apoptosis and enhances proliferation and effector functions [127]. 4-1BB is expressed on lymphocytes from tumor margins of HCC patients [128]. The therapeutic use of antibodies agonist to CD137 showed promising results in HCC animal models [129, 130] and is currently investigated in clinical trials in various malignancies.
CD134 or OX40 is a TNF receptor that has a co-stimulatory function when expressed on T-cells. Targeting OX40 along other immune-related molecules increased CD8 and CD4 T-cell activation in vitro [131] and increased survival in a mouse model bearing HCC [132]. Interestingly, in HCV-induced HCC, OX40 was observed to have an immune inhibitory function when expressed on T-regs [133, 134], suggesting that the effectiveness of targeting this molecule will be partially dependent on the ratio of effector/regulatory T-cells in the tumor microenvironment. OX40 targeting antibodies are currently investigated in clinical trials [135, 136]. The role and therapeutic use of OX40 and other TNF receptors have yet to be explored in the HCC setting.
Summary and future direction
Emerging data described in this article provide evidence to support the clinical investigations of novel immunotherapies in HCC. The final results of the ongoing trials including CheckMate-040 trial is crucial for further combination immunotherapy development in HCC based on efficacy and safety profile. Future research should explore biomarkers for response to immunotherapy in HCC beyond PD-L1 expression mechanisms of resistance to immunotherapy (adaptive immune resistance due to increase suppressor receptors), novel target antigens (neo-antigens), and the concept of locoregional immunoembolization in combination with immune checkpoint inhibitors. Future clinical trials should be designed to study these elements. This can be mostly achieved by incorporating pre- and post-treatment biopsies and by encouraging trials for combinations of therapies based on scientific rationale. Indeed, this is the beginning of a new era for HCC treatment that is likely to expand in the near future.
Acknowledgements
Joseph Obeid and Craig Slingluff Jr. would like to thank the National Cancer Institute for the funding provided for salary support NCI T32 CA163177 and NCI P30 CA044579, respectively.
Abbreviations
- CIK
Cytokine-induced killer
- DFS
Disease-free survival
- GPC-3
Glypican-3
- HCC
Hepatocellular carcinoma
- KC
Kupffer cells
- LAG-3
Lymphocyte activation gene 3
- MDSC
Myeloid-derived suppressor cells
- NKT
Natural killer T
- OS
Overall survival
- PR
Partial response
- SD
Stable disease
- T-reg
T-regulatory
- TACE
Transarterial chemoembolization
- Tim-3
T-cell immunoglobulin mucin 3
- TME
Tumor microenvironment
Compliance with ethical standards
Conflict of interest
Osama Rahma receives research support from Merck and is a speaker for activities supported by educational grants from Bristol-Meyers Squibb and Merck. Craig Slingluff Jr. received material from Merck for an ongoing clinical trial. All other authors have nothing to report.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264 e1–1273 e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korangy F, Hochst B, Manns MP, Greten TF. Immune responses in hepatocellular carcinoma. Dig Dis. 2010;28(1):150–154. doi: 10.1159/000282079. [DOI] [PubMed] [Google Scholar]
- 3.Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: a comprehensive review. World J Hepatol. 2015;7(26):2648–2663. doi: 10.4254/wjh.v7.i26.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res. 2013;19(24):6678–6685. doi: 10.1158/1078-0432.CCR-13-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Khan H, Pillarisetty VG, Katz SC. The prognostic value of liver tumor T cell infiltrates. J Surg Res. 2014;191(1):189–195. doi: 10.1016/j.jss.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215(5102):744–745. doi: 10.1038/215744a0. [DOI] [PubMed] [Google Scholar]
- 8.Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol. 2011;11(7):879–889. doi: 10.1016/j.intimp.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao HQ, Li WM, Lu ZQ, Yao YM. Roles of Tregs in development of hepatocellular carcinoma: a meta-analysis. World J Gastroenterol. 2014;20(24):7971–7978. doi: 10.3748/wjg.v20.i24.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: unique challenges and clinical opportunities. Oncoimmunology. 2012;1(1):48–55. doi: 10.4161/onci.1.1.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res. 2009;69(13):5514–5521. doi: 10.1158/0008-5472.CAN-08-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50(3):799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medina-Echeverz J, Eggert T, Han M, Greten TF. Hepatic myeloid-derived suppressor cells in cancer. Cancer Immunol Immunother. 2015;64(8):931–940. doi: 10.1007/s00262-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells-an overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4(1):e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu T, Gabrilovich DI. Molecular pathways: tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin Cancer Res. 2012;18(18):4877–4882. doi: 10.1158/1078-0432.CCR-11-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Mizukoshi E, Yamashita T, Arai K, Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K, Kaneko S. Myeloid-derived suppressor cells correlate with patient outcomes in hepatic arterial infusion chemotherapy for hepatocellular carcinoma. Cancer Immunol Immunother. 2016;65(6):715–725. doi: 10.1007/s00262-016-1837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, An G, Xie S, Yao Y, Feng G. The clinical and prognostic significance of CD14HLA-DR myeloid-derived suppressor cells in hepatocellular carcinoma patients receiving radiotherapy. Tumour Biol. 2016;37(8):10427–10433. doi: 10.1007/s13277-016-4916-2. [DOI] [PubMed] [Google Scholar]
- 20.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13(3):316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14(10):996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 22.Yan M-L. Inhibition of allogeneic T-cell response by Kupffer cells expressing indoleamine 2,3-dioxygenase. World J Gastroenterol. 2010;16(5):636. doi: 10.3748/wjg.v16.i5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22(2):226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Li J, Salcedo R, Mivechi NF, Trinchieri G, Horuzsko A. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72(16):3977–3986. doi: 10.1158/0008-5472.CAN-12-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Q, Xiao X, Wu Y, Wei Y, Zhu LY, Zhou J, Kuang DM. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41(8):2314–2322. doi: 10.1002/eji.201041282. [DOI] [PubMed] [Google Scholar]
- 27.Yan BC, Gong C, Song J, Krausz T, Tretiakova M, Hyjek E, Al-Ahmadie H, Alves V, Xiao SY, Anders RA, Hart JA. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol. 2010;34(8):1147–1154. doi: 10.1097/PAS.0b013e3181e5dffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang W, Zhang W, Cui W, Li X, Abulajiang G, Li Q. Arginase-1 is a more sensitive marker than HepPar-1 and AFP in differential diagnosis of hepatocellular carcinoma from nonhepatocellular carcinoma. Tumour Biol. 2015;36(5):3881–3886. doi: 10.1007/s13277-014-3030-6. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25(18):2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 31.Piccirillo CA, Tritt M, Sgouroudis E, Albanese A, Pyzik M, Hay V. Control of type 1 autoimmune diabetes by naturally occurring CD4+ CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann N Y Acad Sci. 2005;1051:72–87. doi: 10.1196/annals.1361.048. [DOI] [PubMed] [Google Scholar]
- 32.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45(2):254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Yang Y, Chen Z, Jiang Z, Gu Y, Liu Y, Xu S, Lin C, Pan Z, Zhou W, Cao X. Human hepatocellular carcinoma-infiltrating CD4(+)CD69(+)Foxp3(-) regulatory T cell suppresses T cell response via membrane-bound TGF-beta1. J Mol Med (Berl) 2014;92(5):539–550. doi: 10.1007/s00109-014-1143-4. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 36.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41(4):722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhary B, Elkord E. Regulatory T Cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel) 2016 doi: 10.3390/vaccines4030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauer AV, Brigida I, Carriglio N, Hernandez RJ, Scaramuzza S, Clavenna D, Sanvito F, Poliani PL, Gagliani N, Carlucci F, Tabucchi A, Roncarolo MG, Traggiai E, Villa A, Aiuti A. Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood. 2012;119(6):1428–1439. doi: 10.1182/blood-2011-07-366781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101(28):10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang FS. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129(3):428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65(6):2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 42.Zhang HH, Mei MH, Fei R, Liu F, Wang JH, Liao WJ, Qin LL, Wei L, Chen HS. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat. 2010;17(Suppl 1):34–43. doi: 10.1111/j.1365-2893.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 43.Miroux C, Vausselin T, Delhem N. Regulatory T cells in HBV and HCV liver diseases: implication of regulatory T lymphocytes in the control of immune response. Expert Opin Biol Ther. 2010;10(11):1563–1572. doi: 10.1517/14712598.2010.529125. [DOI] [PubMed] [Google Scholar]
- 44.Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29(3):1817–1826. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13(3):902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, Shibata K, Ohta M, Kitano S, Mori M. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34(2):173–179. doi: 10.1016/j.ejso.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Liu T, Tang W, Deng B, Chen Y, Zhu J, Shen X. Hepatocellular carcinoma cells induce regulatory T Cells and lead to poor prognosis via production of transforming growth factor-beta1. Cell Physiol Biochem. 2016;38(1):306–318. doi: 10.1159/000438631. [DOI] [PubMed] [Google Scholar]
- 48.Yang ZQ, Yang ZY, Zhang LD, Ping B, Wang SG, Ma KS, Li XW, Dong JH. Increased liver-infiltrating CD8+ FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol. 2010;71(12):1180–1186. doi: 10.1016/j.humimm.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Salgado R, Denkert C, Campbell C, Savas P, Nucifero P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J, Piccart-Gebhart MJ, Michiels S, Bradbury I, Sotiriou C, Loi S. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a Secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O’Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D’Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL., Jr Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun C, Xu J, Song J, Liu C, Wang J, Weng C, Sun H, Wei H, Xiao W, Sun R, Tian Z. The predictive value of centre tumour CD8+ T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget. 2015;6(34):35602–35615. doi: 10.18632/oncotarget.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45(4):451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 55.Cicinnati VR, Zhang X, Yu Z, Ferencik S, Schmitz KJ, Dworacki G, Kaczmarek E, Oldhafer K, Frilling A, Baba HA, Schmid KW, Grosse-Wilde H, Broelsch CE, DeLeo AB, Gerken G, Beckebaum S. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer. 2006;119(12):2851–2860. doi: 10.1002/ijc.22251. [DOI] [PubMed] [Google Scholar]
- 56.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS, Gnjatic S, Ritter G, Simpson AJ, Old LJ, Chen WF. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin Cancer Res. 2004;10(20):6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 57.Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, Kagaya T, Yamashita T, Honda M, Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53(4):1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 58.Sayem MA, Tomita Y, Yuno A, Hirayama M, Irie A, Tsukamoto H, Senju S, Yuba E, Yoshikawa T, Kono K, Nakatsura T, Nishimura Y. Identification of glypican-3-derived long peptides activating both CD8 and CD4 T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology. 2016;5(1):e1062209. doi: 10.1080/2162402X.2015.1062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Luan W, Warren L, Fiel MI, Blank S, Kadri H, Mandeli J, Hiotis SP. Prognostic role of immune cells in hepatitis B-associated hepatocellular carcinoma following surgical resection depends on their localization and tumor size. J Immunother. 2016;39(1):36–44. doi: 10.1097/CJI.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 60.Huang Y, Wang FM, Wang T, Wang YJ, Zhu ZY, Gao YT, Du Z. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion. 2012;86(4):329–337. doi: 10.1159/000342801. [DOI] [PubMed] [Google Scholar]
- 61.Wang F, Jing X, Li G, Wang T, Yang B, Zhu Z, Gao Y, Zhang Q, Yang Y, Wang Y, Wang P, Du Z. Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int. 2012;32(4):644–655. doi: 10.1111/j.1478-3231.2011.02675.x. [DOI] [PubMed] [Google Scholar]
- 62.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu J, Zhang Z, Zhou L, Qi Z, Xing S, Lv J, Shi J, Fu B, Liu Z, Zhang JY, Jin L, Zhao Y, Lau GK, Zhao J, Wang FS. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58(1):139–149. doi: 10.1002/hep.26054. [DOI] [PubMed] [Google Scholar]
- 65.Cao DY, Yang JY, Yue SQ, Tao KS, Song ZS, Wang DS, Yang YL, Dou KF. Comparative analysis of DC fused with allogeneic hepatocellular carcinoma cell line HepG2 and autologous tumor cells as potential cancer vaccines against hepatocellular carcinoma. Cell Immunol. 2009;259(1):13–20. doi: 10.1016/j.cellimm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Shibolet O, Alper R, Zlotogarov L, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. NKT and CD8 lymphocytes mediate suppression of hepatocellular carcinoma growth via tumor antigen-pulsed dendritic cells. Int J Cancer. 2003;106(2):236–243. doi: 10.1002/ijc.11201. [DOI] [PubMed] [Google Scholar]
- 67.Zhang CD, Wang JN, Sui BQ, Zeng YJ, Chen JQ, Dai DQ. Prognostic and predictive model for stage II colon cancer patients with nonemergent surgery: who should receive adjuvant chemotherapy? Medicine. 2016;95(1):e2190. doi: 10.1097/MD.0000000000002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66(22):11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 70.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vermijlen D, Seynaeve C, Luo D, Kruhoffer M, Eizirik DL, Orntoft TF, Wisse E. High-density oligonucleotide array analysis reveals extensive differences between freshly isolated blood and hepatic natural killer cells. Eur J Immunol. 2004;34(9):2529–2540. doi: 10.1002/eji.200324712. [DOI] [PubMed] [Google Scholar]
- 72.Gong J, Fang L, Liu R, Wang Y, Xing J, Chen Y, Zhuang R, Zhang Y, Zhang C, Yang A, Zhang X, Jin B, Chen L. UPR decreases CD226 ligand CD155 expression and sensitivity to NK cell-mediated cytotoxicity in hepatoma cells. Eur J Immunol. 2014;44(12):3758–3767. doi: 10.1002/eji.201444574. [DOI] [PubMed] [Google Scholar]
- 73.Qu P, Huang X, Zhou X, Lu Z, Liu F, Shi Z, Lu L, Wu Y, Chen Y. Loss of CD155 expression predicts poor prognosis in hepatocellular carcinoma. Histopathology. 2015;66(5):706–714. doi: 10.1111/his.12584. [DOI] [PubMed] [Google Scholar]
- 74.Wongkajornsilp A, Numchaisermsuk N, Sa-Ngiamsuntorn K, Akarasereenont P, Wamanuttajinda V, Kasetsinsombat K, Duangsa-Ard S, Laohapan T, Maneechotesuwan K. Effects of the Ayurved Siriraj Wattana recipe on functional and phenotypic characterization of cytokine-induced killer cells and dendritic cells in vitro. BMC Complement Altern Med. 2016;16(1):489. doi: 10.1186/s12906-016-1480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Margalit M, Shibolet O, Klein A, Elinav E, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma by transplantation of ex vivo immune-modulated NKT lymphocytes. Int J Cancer. 2005;115(3):443–449. doi: 10.1002/ijc.20889. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, Lau GK, Fu YX, Wang FS. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134(7):1938–1949. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 77.Larrubia JR. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J Gastroenterol. 2009;15(41):5129. doi: 10.3748/wjg.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, Yang YP, Tien P, Wang FS. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128(4):887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 79.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 80.Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, Yamashita Y, Yoshizumi T, Ikegami T, Soejima Y, Harada M, Aishima S, Oda Y, Shirabe K, Maehara Y. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol. 2015;50(1):65–75. doi: 10.1007/s00535-014-0933-3. [DOI] [PubMed] [Google Scholar]
- 81.Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA. The role of neoantigens in the response to immune checkpoint blockade. Int Immunol. 2016;28(8):411–419. doi: 10.1093/intimm/dxw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, Zhou W, Cao X. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59(2):567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 83.Sierro S, Romero P, Speiser DE. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets. 2011;15(1):91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 84.Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, Hartmann D, Black RA, Rossi JJ, Blobel CP, Dempsey PJ, Workman CJ, Vignali DA. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26(2):494–504. doi: 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174(2):688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 86.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res. 2015;3(4):412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169(10):5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 89.Li FJ, Zhang Y, Jin GX, Yao L, Wu DQ. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8(+) T cell in HCC patients. Immunol Lett. 2013;150(1–2):116–122. doi: 10.1016/j.imlet.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 90.Ikushima H, Miyazono K. Biology of transforming growth factor-beta signaling. Curr Pharm Biotechnol. 2011;12(12):2099–2107. doi: 10.2174/138920111798808419. [DOI] [PubMed] [Google Scholar]
- 91.Lin S, Xie J, Gong T, Shi S, Zhang T, Fu N, Ye L, Wang M, Lin Y. TGFbeta signalling pathway regulates angiogenesis by endothelial cells, in an adipose-derived stromal cell/endothelial cell co-culture 3D gel model. Cell Prolif. 2015;48(6):729–737. doi: 10.1111/cpr.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mouri H, Sakaguchi K, Sawayama T, Senoh T, Ohta T, Nishimura M, Fujiwara A, Terao M, Shiratori Y, Tsuji T. Suppressive effects of transforming growth factor-beta1 produced by hepatocellular carcinoma cell lines on interferon-gamma production by peripheral blood mononuclear cells. Acta Med Okayama. 2002;56(6):309–315. doi: 10.18926/AMO/31688. [DOI] [PubMed] [Google Scholar]
- 93.Park NR, Cha JH, Jang JW, Bae SH, Jang B, Kim JH, Hur W, Choi JY, Yoon SK. Synergistic effects of CD44 and TGF-beta1 through AKT/GSK-3beta/beta-catenin signaling during epithelial-mesenchymal transition in liver cancer cells. Biochem Biophys Res Commun. 2016;477(4):568–574. doi: 10.1016/j.bbrc.2016.06.077. [DOI] [PubMed] [Google Scholar]
- 94.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, Fan ST. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12(18):5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 95.Ji F, Fu SJ, Shen SL, Zhang LJ, Cao QH, Li SQ, Peng BG, Liang LJ, Hua YP. The prognostic value of combined TGF-beta1 and ELF in hepatocellular carcinoma. BMC Cancer. 2015;15:116. doi: 10.1186/s12885-015-1127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin TH, Shao YY, Chan SY, Huang CY, Hsu CH, Cheng AL. High serum transforming growth factor-beta1 levels predict outcome in hepatocellular carcinoma patients treated with sorafenib. Clin Cancer Res. 2015;21(16):3678–3684. doi: 10.1158/1078-0432.CCR-14-1954. [DOI] [PubMed] [Google Scholar]
- 97.Toubaji A, Achtar M, Provenzano M, Herrin VE, Behrens R, Hamilton M, Bernstein S, Venzon D, Gause B, Marincola F, Khleif SN. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother. 2008;57(9):1413–1420. doi: 10.1007/s00262-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan childhood hepatoma study group. N Engl J Med. 1997;336(26):1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 99.Wang XG, Revskaya E, Bryan RA, Strickler HD, Burk RD, Casadevall A, Dadachova E. Treating cancer as an infectious disease–viral antigens as novel targets for treatment and potential prevention of tumors of viral etiology. PLoS One. 2007;2(10):e1114. doi: 10.1371/journal.pone.0001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahma OE, Khleif SN. Therapeutic vaccines for gastrointestinal cancers. Gastroenterol Hepatol (N Y) 2011;7(8):517–564. [PMC free article] [PubMed] [Google Scholar]
- 101.Sun Z, Zhu Y, Xia J, Sawakami T, Kokudo N, Zhang N. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. Biosci Trends. 2015;10(2):85–91. doi: 10.5582/bst.2015.01128. [DOI] [PubMed] [Google Scholar]
- 102.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125(1):89–97. doi: 10.1016/S0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 103.Fu SJ, Qi CY, Xiao WK, Li SQ, Peng BG, Liang LJ. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery. 2013;154(3):536–544. doi: 10.1016/j.surg.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 104.Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao K, Uesaka K, Furuse J, Kinoshita T, Nakatsura T. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18(13):3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 105.Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M, Kinoshita T, Ikeda M, Nakachi K, Yamazaki N, Mizuno S, Takayama T, Yamao K, Uesaka K, Furuse J, Endo I, Nakatsura T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5):e1129483. doi: 10.1080/2162402X.2015.1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 107.Xu L, Wang J, Kim Y, Shuang ZY, Zhang YJ, Lao XM, Li YQ, Chen MS, Pawlik TM, Xia JC, Li SP, Lau WY. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology. 2016;5(3):e1083671. doi: 10.1080/2162402X.2015.1083671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerry A, Sanderson J, Maroto M, Ferronha T, Ranganathan S, Norry E, Pandite L, Amado RG, Jakobsen BK (2016) Targeting alpha fetoprotein with TCR engineered T cells in HCC. ASCO Annual Meeting 2016. J Clin Oncol 34 (suppl; abstr 3051; Abstract)
- 109.Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ, Wang QJ, Huang LX, He J, Chen SP, Ke ML, Wu PH, Chen MS, Li SP, Xia JC, Zeng YX. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20(13):4305–4311. doi: 10.1245/s10434-013-3144-x. [DOI] [PubMed] [Google Scholar]
- 110.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383–1391.e6. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 111.Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1(1):11. doi: 10.1186/2162-3619-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sangro B, Gomez-Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Perez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 113.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sangro B, Park J-W, Cruz CMD, Anderson J, Lang L, Neely J, Shaw JW, Cheng A-L (2016) A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. Annual Meeting, 2016. J Clin Oncol 34 (suppl; abstr TPS4147; Abstract)
- 115.Faivre SJ, Santoro A, Kelley RK, Merle P, Gane E, Douillard J-Y, Waldschmidt D, Mulcahy MF, Costentin C, Minguez B, Papappicco P, Gueorguieva I, Cleverly A, Desaiah D, Lahn MM, Ameryckx S, Benhadji KA, Raymond E, Giannelli G (2014) A phase 2 study of a novel transforming growth factor-beta (TGF-β1) receptor I kinase inhibitor, LY2157299 monohydrate (LY), in patients with advanced hepatocellular carcinoma (HCC). Gastrointestinal Cancers Symposium, 2014. J Clin Oncol 32, 2014 (suppl 3; abstr LBA173; Abstract)
- 116.Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, Cerioni S, Fagnoni F, Soliani P, Ferrari C, Missale G. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138(5):1931–1942. doi: 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 117.Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 118.Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I, Kohno S. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43(4):575–577. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakanishi M, Chuma M, Hige S, Asaka M. Abscopal effect on hepatocellular carcinoma. Am J Gastroenterol. 2008;103(5):1320–1321. doi: 10.1111/j.1572-0241.2007.01782_13.x. [DOI] [PubMed] [Google Scholar]
- 120.Duffy AG, Makarova-Rusher OV, Pratt D, Kleiner DE, Ulahannan S, Mabry D, Fioravanti S, Walker M, Carey S, Figg WD, Steinberg SM, Anderson V, Levy E, Krishnasamy V, Wood BJ, Greten TF (2016) Tremelimumab: a monoclonal antibody against CTLA-4—In combination with subtotal ablation (trans catheter arterial chemoembolization (TACE), radiofrequency ablation (RFA) or cryoablation) in patients with hepatocellular carcinoma (HCC) and biliary tract carcinoma (BTC). ASCO Annual Meeting, 2016. J Clin Oncol 34 (suppl; abstr 4073; Abstract)
- 121.Greten TF, Ormandy LA, Fikuart A, Hochst B, Henschen S, Horning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33(2):211–218. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 122.De la Torre AN, Castaneda I, Contractor S, Salazar AM (2015) Use of low-dose cyclophosphaminde followed by low-dose tumoral radiation, intratumoral poly-ICLC combined with local-regional therapy, followed by systemic immune boosting with intramuscular poly-ICLC in patients with cancers of the liver. Gastrointestinal Cancers Symposium, 2015. J Clin Oncol 33 (suppl 3; abstr 327; Abstract)
- 123.Chen ML, Yan BS, Lu WC, Chen MH, Yu SL, Yang PC, Cheng AL. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int J Cancer. 2014;134(2):319–331. doi: 10.1002/ijc.28362. [DOI] [PubMed] [Google Scholar]
- 124.Heine A, Schilling J, Grunwald B, Kruger A, Gevensleben H, Held SA, Garbi N, Kurts C, Brossart P, Knolle P, Diehl L, Hochst B. The induction of human myeloid derived suppressor cells through hepatic stellate cells is dose-dependently inhibited by the tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but not sunitinib. Cancer Immunol Immunother. 2016;65(3):273–282. doi: 10.1007/s00262-015-1790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abou-Alfa GK, Sangro B, Morse M, Zhu AX, Kim RD, Cheng A-L, Kudo M, Kang Y-K, Chan SL, Antal J, Boice J, Xiao F, Morris SR, Bendell J (2016) Phase 1/2 study of durvalumab and tremelimumab as monotherapy and in combination in patients with unresectable hepatocellular carcinoma (HCC). ASCO Annual Meeting, 2016. J Clin Oncol 35 (suppl; abstr 4073; Abstract)
- 126.Courau T, Nehar-Belaid D, Florez L, Levacher B, Vazquez T, Brimaud F, Bellier B, Klatzmann D. TGF-beta and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight. 2016;1(9):e85974. doi: 10.1172/jci.insight.85974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilikueta A, Etxeberria I, Bolanos E, Lang V, Rodriguez M, Aznar MA, Jure-Kunkel M, Melero I. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur J Immunol. 2016;46(3):513–522. doi: 10.1002/eji.201445388. [DOI] [PubMed] [Google Scholar]
- 128.Wan YL, Zheng SS, Zhao ZC, Li MW, Jia CK, Zhang H. Expression of co-stimulator 4-1BB molecule in hepatocellular carcinoma and adjacent non-tumor liver tissue, and its possible role in tumor immunity. World J Gastroenterol. 2004;10(2):195–199. doi: 10.3748/wjg.v10.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gauttier V, Judor JP, Le Guen V, Cany J, Ferry N, Conchon S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer. 2014;135(12):2857–2867. doi: 10.1002/ijc.28943. [DOI] [PubMed] [Google Scholar]
- 130.Xiao H, Huang B, Yuan Y, Li D, Han LF, Liu Y, Gong W, Wu FH, Zhang GM, Feng ZH. Soluble PD-1 facilitates 4-1BBL-triggered antitumor immunity against murine H22 hepatocarcinoma in vivo. Clin Cancer Res. 2007;13(6):1823–1830. doi: 10.1158/1078-0432.CCR-06-2154. [DOI] [PubMed] [Google Scholar]
- 131.Chen M, Ouyang H, Zhou S, Li J, Ye Y. Effect of PLGA nanoparticles conjugated with anti-OX40/anti-AFP mAbs on cytotoxicity of CTL cells against hepatocellular carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30(4):337–341. [PubMed] [Google Scholar]
- 132.Morales-Kastresana A, Sanmamed MF, Rodriguez I, Palazon A, Martinez-Forero I, Labiano S, Hervas-Stubbs S, Sangro B, Ochoa C, Rouzaut A, Azpilikueta A, Bolanos E, Jure-Kunkel M, Gutgemann I, Melero I. Combined immunostimulatory monoclonal antibodies extend survival in an aggressive transgenic hepatocellular carcinoma mouse model. Clin Cancer Res. 2013;19(22):6151–6162. doi: 10.1158/1078-0432.CCR-13-1189. [DOI] [PubMed] [Google Scholar]
- 133.Piconese S, Timperi E, Pacella I, Schinzari V, Tripodo C, Rossi M, Guglielmo N, Mennini G, Grazi GL, Di Filippo S, Brozzetti S, Fazzi K, Antonelli G, Lozzi MA, Sanchez M, Barnaba V. Human OX40 tunes the function of regulatory T cells in tumor and nontumor areas of hepatitis C virus-infected liver tissue. Hepatology. 2014;60(5):1494–1507. doi: 10.1002/hep.27188. [DOI] [PubMed] [Google Scholar]
- 134.Wang XF, Korangy F. Intrahepatic landscape of regulatory T-cell subsets in chronically HCV-infected patients with cirrhosis and HCC. Hepatology. 2014;60(5):1461–1462. doi: 10.1002/hep.27271. [DOI] [PubMed] [Google Scholar]
- 135.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 136.Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I. Agonists of Co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640–655. doi: 10.1053/j.seminoncol.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 137.Heo J, Breitbach CJ, Moon A, Kim CW, Patt R, Kim MK, Lee YK, Oh SY, Woo HY, Parato K, Rintoul J, Falls T, Hickman T, Rhee BG, Bell JC, Kirn DH, Hwang TH. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther. 2011;19(6):1170–1179. doi: 10.1038/mt.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sangro B, Melero I, Yau TC, Hsu C, Kudo M, Crocenzi TS, Kim T-Y, Choo S, Trojan J, Meyer T, Kang Y-K, Anderson J, Cruz CMD, Lang L, Neely J, El-Khoueiry B (2016) A Safety and antitumor activity of nivolumab (nivo) in patients (pts) with advanced hepatocellular carcinoma (HCC): Interim analysis of dose-expansion cohorts from the phase 1/2 CheckMate-040 study. ASCO Annual Meeting, 2016. J Clin Oncol 34 (suppl; abstr 4078; Abstract)