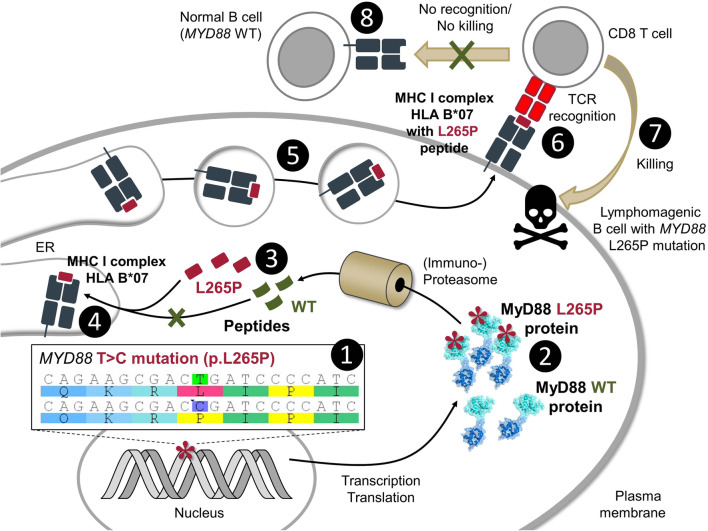
Fig. 2.
Presentation of peptides containing L265P mutations as T-cell neo-epitopes. Due to a mostly heterozygous T to C transition (1), L265P-mutated proteins are synthesized alongside WT protein (2). As with most cellular proteins, both MyD88 forms will be subject to immuno-proteosomal degradation into peptides (3) that are typically loaded onto MHC class I (4) and II complexes (not shown) by different cellular pathways (not shown in detail). For certain HLA types, the amino acid sequence of the WT peptide will not be compatible with the requirements for efficient HLA-binding compared to L265P-containing peptides, e.g., when the anchor position is affected. Following trafficking of loaded MHC I-peptide complexes from the ER to the cell surface (5), the MHC I-peptide complex is sampled by T cells via their T-cell receptor (TCR) (6). Since L265P-containing peptides represent neo-antigens that were not negatively selected during T-cell development, certain CD8 T cells with specific TCRs may bind such MHC I-L265P-peptide complexes and be activated for killing (7), whereas non-mutated normal B cells or other tissue cells are not recognized (8)