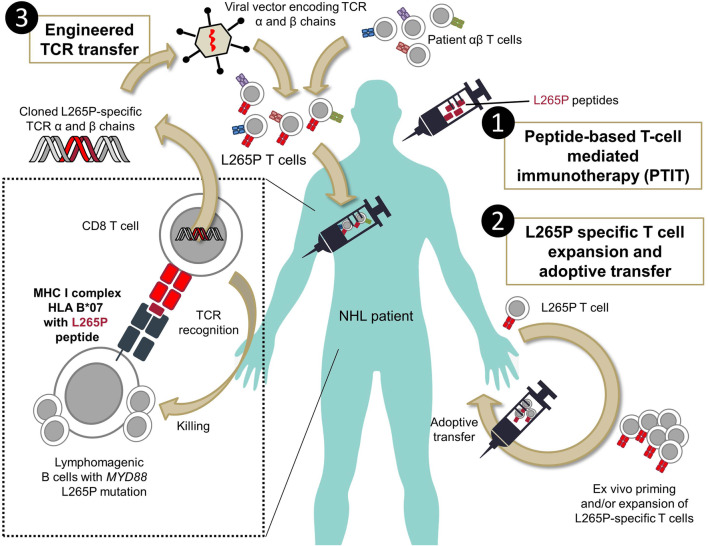
Fig. 3.
Exploitation of L265P mutations for T-cell-based immunotherapy. The figure shows three conceivable approaches for exploiting L265P mutations in T-cell-based immunotherapies: (1) patients with the appropriate HLA types and confirmed MYD88 mutation are vaccinated with MyD88 L265P-derived peptides to prime specific T cells in the patient. (2) Where this is not possible or ineffective, T cells can either be primed from patient PBMC entirely ex vivo, or low numbers of specific T cells be expanded ex vivo under GMP conditions. (3) Alternatively, polyclonal T cells from the patient are virally transduced with TCR sequences. These TCRs could be identified and cloned beforehand from T cells primed with L265P peptides affinity matured in vitro before engraftment into the viral vector. For each HLA type, a single defined TCR sequence pair (for α and β chains) would need to be generated once to be applied to all patients with L265P mutation bearing this HLA-type. A combination of different viruses to exploit all possible L265P-conducive HLA alleles in the patient would be feasible. Following viral transduction of the polyclonal patient T cells, the engineered T cells, which express an HLA-L265P-restricted TCR in addition to their original/cognate TCR will be adoptively transferred back to the patient