Abstract
Tumour-associated macrophages (TAMs) are the key components in the tumour microenvironment. TAMs have two major subtypes, M1 and M2. M1 macrophages are tumour inhibitory, while M2 macrophages are tumour promotive. Repolarising TAMs from M2 to M1 is a promising strategy in cancer treatment. M1 and M2 macrophages were generated from murine bone marrow-derived macrophages (BMDMs). We found that chloroquine (CQ), an autophagy inhibitor, was able to repolarise M2 macrophages to the anti-tumour M1 phenotype. The repolarised macrophages demonstrated higher phagocytotic activity towards Hep-2 laryngeal tumour cells and re-sensitised Hep-2 cells to cisplatin (CDDP) treatment in vitro. While CQ did not demonstrate cytotoxicity to Hep-2 cells in vitro, CQ treatment reduced Hep-2 laryngeal tumour growth in vivo and improved CDDP treatment outcomes. It seems that CQ-induced M2-to-M1 macrophage repolarisation played an important role in tumour growth inhibition, and the CQ/CDDP combined therapy might have clinical potential in laryngeal cancer treatment.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02415-8) contains supplementary material, which is available to authorized users.
Keywords: Chloroquine (CQ), Tumour-associated macrophages (TAMs), Autophagy inhibition, Macrophage repolarisation, Hep-2 laryngeal cancer
Introduction
Tumour-associated macrophages (TAMs) are one of the major cellular components and key regulators in the tumour microenvironment. They are involved in tumour growth, angiogenesis and lymphangiogenesis, tumour invasion, metastasis and immunosuppression of many cancers, including laryngeal cancer [1–3]. In the last decade, TAMs were found to contribute to the chemotherapy resistance [4]. Chemotherapeutic agents such as paclitaxel and platinum disrupt the mitotic process and induce apoptosis of the cancerous cells. The death of the tumour cells would stimulate TAM expansion and recruit their circulating precursors, and the proliferated TAMs would secrete cytokines to accelerate cancer stem cell replication and suppress apoptosis [5]. It has been shown that in colon cancer, pancreatic cancer and breast cancer, targeting TAMs could suppress tumour progression and improve chemotherapy efficacy [5–7].
Macrophages are immune cells that have a high degree of phenotypical and functional heterogeneity, but they are often simplified into two subtypes. M1 macrophages are ‘classically’ activated, pro-inflammatory and tumour-inhibitory, while M2 macrophages are ‘alternatively’ activated, anti-inflammatory and tumour promotive [2, 8]. The composition of macrophage subtypes in a tumour tissue determines the inhibitory or promotive role of TAMs. In many cancers, TAMs are dominated by M2 macrophages and thus play a tumour-promotive role [1, 3]. Hence, re-educating TAMs from M2 to M1 phenotype is a promising anticancer strategy [9–11].
Autophagy is an essential cellular function for macrophages [12–14]. This process is not only involved in intracellular quality control and metabolic adaptation, but also crucial for cell differentiation and remodelling [14, 15]. It has been revealed that the down-regulation of autophagy level limits the M2 macrophage differentiation [16]. Based on this knowledge, herein, we hypothesised that chloroquine (CQ), an autophagy inhibitor, can repolarise TAMs from M2 macrophages to M1 phenotype. First, we proved that CQ could repolarise M2 macrophages to M1 phenotype in vitro. Then, the effects of repolarised macrophages on Hep-2 laryngeal tumour cells and cisplatin (CDDP) resistance were examined. Finally, we assessed the effects of CQ treatment in vivo. It was demonstrated that CQ could reduce laryngeal tumour growth and effectively enhance CDDP sensitivity, and TAMs were successfully repolarised during this process.
Methods
Mice
C57BL/6 mice and nu/nu nude mice were used for macrophage generation and tumour transplantation, respectively. Mice were maintained in pathogen-free facilities.
Cell models
Murine bone marrow-derived macrophages (BMDMs) were used to generate M1 and M2 macrophages. Murine BMDMs were isolated following the published procedure [17]. The bone marrow was extracted from femur and tibia of 6 to 8-week-old C57BL/6 mice and was dissociated by passing through 10 mL syringe with 26 G needle. The cells were isolated by passing through a 40 μm strainer, and the red blood cells were lysed by incubating in 0.8% NH4Cl (Sigma, St. Louis, MO, USA) solution on ice for 10 min. The remaining cells were then grown in BMDM growth medium (Dulbecco’s modified Eagle medium, DMEM, Gibco), 10% fetal bovine serum (FBS, Gibco), 100 IU penicillin (Sigma, St. Louis, MO, USA), 100 μg/mL streptomycin (Sigma) and 10 ng/mL recombinant murine M-CSF (Invitrogen, Waltham, MA, USA) at 1 × 106 cells/mL for 7 days to amplify the macrophages. Human laryngeal cancer Hep-2 cell line and GFP-labelled Hep-2-GFP cells were kept in DMEM supplemented with 10% FBS. All the cell lines were tested for mycoplasma contamination regularly. All the cells were incubated in indicated medium at 37 °C in an atmosphere containing 5% CO2. All the mediums were replenished every 2 days.
Macrophage polarisation in vitro
Murine BMDMs were grown in the BMDM growth medium at 37 °C and 5% CO2 to maintain the M0 state. The cells were induced with 50 ng/mL interferon-γ (IFN-γ) (Thermo, Waltham, MA, USA) and 100 ng/mL lipopolysaccharide (LPS) (Sigma) for M1 polarisation and 10 ng/mL interleukin-4 (IL-4) (Thermo) for M2 polarisation [17]. The cells were induced for 24 h, after which the media were aspirated, and cells were washed with cold phosphate-buffered saline (PBS) and collected for transcriptional analysis. To characterise the polarisation state, the transcription levels of the genes encoding arginase 1 (Arg1), mannose receptor 1 (Mrc1I), IL-12 (Il12) and nitric oxide synthase (Nos2) were measured using quantitative real-time PCR (qRT-PCR).
Macrophage repolarisation in vitro
M2 macrophages generated from murine BMDMs were treated with 20 μM CQ (Sigma) dissolved in PBS for 24 h to induce M2-to-M1 repolarisation. PBS was used to treat the control group for 24 h. The media were aspirated, and resultant cells were washed with cold PBS and collected for qRT-PCR, western blot and flow cytometry analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed to analyse the phenotype of the macrophages. RNA extraction was conducted using RNeasy kit (Fermentas Life Sciences). cDNA synthesis was conducted using Invitrogen SuperScript™ VILO™ cDNA synthesis kit. Primers for mouse Arg1, Mrc1, Il12 and Nos2 were purchased from Sino Biological. qRT-PCR was performed using 7300 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). Agarose gel electrophoresis was performed to confirm the right PCR products. The relative amounts of target mRNAs in the experimental groups were normalised to the amounts of target mRNAs in M0 macrophages.
Western blotting
Western blot was used for autophagy level analysis and macrophages phenotyping. The harvested cells were lysed on ice with the lysis buffer (Sigma) supplemented with the protease inhibitor cocktail (Sigma). An equal volume of 2 × sodium dodecyl sulphate sample buffer (Sigma) was added and the lysates were boiled for 10 min. Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and were transferred to blotting membrane using Invitrogen iBlot® 2 gel transfer device. The membrane was blocked with 5% skimmed milk (Sigma) at 4 °C overnight, and then probed with anti-LC3-I, anti-LC3-II, anti-GAPDH, anti-ARG1, anti-MRC1, anti-IL12 or anti-NOS2 primary antibodies (Abcam, Cambridge, UK) for 2 h at room temperature. After being washed, the membrane was incubated with horseradish peroxide-conjugated goat-anti-mouse IgG secondary antibody (Boster, Wuhan, China) for 1 h at room temperature. The membrane was then washed again and visualised using chemiluminescence kit (SuperSignal® West Pico Chemiluminescent Substrate from Thermo Scientific). The densitometric analysis was conducted using ImageJ (version 1.47).
In vitro phagocytosis assay
In vitro phagocytosis assay was conducted by co-culturing the macrophages with Hep-2-GFP cells following the protocol of [18]. M2 macrophages derived from BMDMs were pre-treated with 20 μM CQ or PBS for 24 h. Hep-2-GFP cells were incubated in 12-well plates at 5 × 105 cells/well for 2 h to allow for adherence, after which 1.5 × 106 macrophages were added to each well, and cells were co-cultured for 24 h. After incubation, media were aspirated, and cells were washed with cold PBS and resuspended in Hanks’ balanced salt solution (Sigma) supplemented with 1% bovine serum albumin (Sigma). Flow cytometry was used to detect the phagocytosis of Hep-2-GFP cells by the macrophages. Phagocytosis was calculated as the percentage of GFP+ cells among F4/80+ macrophages.
In vitro drug sensitivity assay
In vitro drug sensitivity assay was conducted by culturing Hep-2 cells with macrophage-conditioned media during CDDP (Sigma) treatment. The BMDM-derived M2 macrophages were pre-treated with 20 μM CQ or PBS for 24 h, and the conditioned media (CM) were collected. The BMDM-derived M1 macrophages were pre-treated with PBS for 24 h, and the conditioned media (CM) were also collected. Hep-2 cells were incubated in 12-well plates at 5 × 105 cells/well in five different media: BMDM growth medium, BMDM growth medium supplemented with CDDP (2.5 μM); PBS-treated M2-CM supplemented with CDDP; CQ-treated M2-CM supplemented with CDDP, and M1-CM supplemented with CDDP. Cells were incubated for 48 h before being analysed by flow cytometry to detect the apoptotic cells.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay
Hep-2 cells were inoculated at 1 × 105 cells per mL in 96-well plate in BMDM growth medium supplemented with PBS or 20 μM CQ. After the treatment for 48 h, the media were discarded and serum-free media (ThermoFisher) were added. MTT assay was then performed to analyse the cell viability following the manufacturer’s instruction (MTT Cell Viability Assay Kit, Abnova, Taiwan). OD570 nm was measured in this assay. The viable cells were calculated based on the absorbance-cell number titration.
Tumour growth models
Hep-2 laryngeal tumour models were established on 6–8 weeks old male nu/nu mice. Tumour growth was initiated by subcutaneous injection of 2 × 106 Hep-2 cells and 10% Matrigel (BD Biosciences, Beijing, China) mixture under the right back skin. The sizes of tumours were measured every 2 days using calliper. The volume of the tumour was calculated by length × width2/2. Mice started to receive treatment when the tumour reached approximate 50 mm3. Mice were divided into four groups (n = 8 for each group) and received injections of: CQ (10 mg/kg mouse weight), CDDP (2.5 mg/kg mouse weight), CQ + CDDP (CQ 10 mg/kg + CDDP 2.5 mg/kg) or PBS. This dosage of CQ, if converted to human, would be about 1.59 mg/kg human weight [19]. The treatment was given every 3 days for five times. Mice were killed 21 days after the first injection, and the tumours were surgically sectioned for tumour weight measurement and subsequent assays. The mean volume and mean weight of the tumours of each group were used to plot the tumour growth curves.
Immunohistochemistry and immunofluorescence
PCNA assay and TUNEL assay were used to detect the proliferating and apoptotic cells in the tumour sections, respectively. Tumours that received various treatments were frozen sectioned, fixed with paraformaldehyde (Sigma) and embedded in paraffin (Sigma). PCNA assay was conducted using PCNA ELISA Kit (4A Biotech, Beijing, China) following the manufacturer’s instructions. TUNEL assay was conducted using One Step TUNEL apoptosis assay kit (Beyotime, Shanghai, China). The tumour sections were washed with PBS and incubated with proteinase K (Sigma) at room temperature for 3 min before being stained following the manufacturer’s protocol. The imaging was performed using fluorescent microscope (Olympus, Tokyo, Japan), and the cell counting was performed using ImageJ.
Autophagy detection
Autophagy activity was compared between macrophages and between tumours using western blot against lipidated microtubule-associated protein 1 light chain 3 (LC3-II), an indicator of active autophagy [20]. LC3-I, the soluble form and precursor of LC3-II, was also subject to the measurement. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was visualised as loading control. LC3-II levels in the treated cells and tumours were normalised to the cells and tumours in the control groups.
Flow cytometry
For the characterisation of macrophages from the cell culture, cells were stained for M1 marker MHCII using anti-MHCII-APC (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and M2 marker CD206 using anti-CD206-APC (Santa Cruz Biotechnology). For in vitro phagocytosis assay, cells were probed with anti-F4/80 primary antibody (Santa Cruz Biotechnology) and then allophycocyanin (APC)-conjugated secondary antibody (Boster, Wuhan, China). For in vitro drug sensitivity assay, cells were stained with annexin V-FICT (BD Biosciences) and propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature for 15 min in the dark. For the characterisation of macrophages from the tumours, the tumours were minced, and single cell suspensions were prepared. The cells were first incubated with Fc receptor blocker (Innovex Biosciences, Middleton, WI, USA) for 10 min on ice, before being probed with anti-F4/80, anti-CD11b, anti-CD206 and anti-MHCII primary antibodies (Santa Cruz Biotechnology) and tetramethylrhodamine isothiocyanate (TRITC) goat-anti-mouse IgG or fluorescein isothiocyanate (FITC) goat-anti-mouse IgG secondary antibodies (Boster, Wuhan, China). All the stained cells were analysed by flow cytometry (BD Biosciences, San Diego, CA, USA). Data were analysed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistics
Statistical analysis was performed using SPSS 17.0 software. All cell experiments were conducted in triplicates. The data were representative of three biological replicates and presented as mean ± standard deviation (SD) in a single experiment if not indicated otherwise. The Student’s t test was performed to determine the significance for differences between two groups. One-way ANOVA was used to compare the differences between multiple groups. Significance was determined as p < 0.05 (*p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001).
Results
M1 and M2 macrophages polarisation in vitro
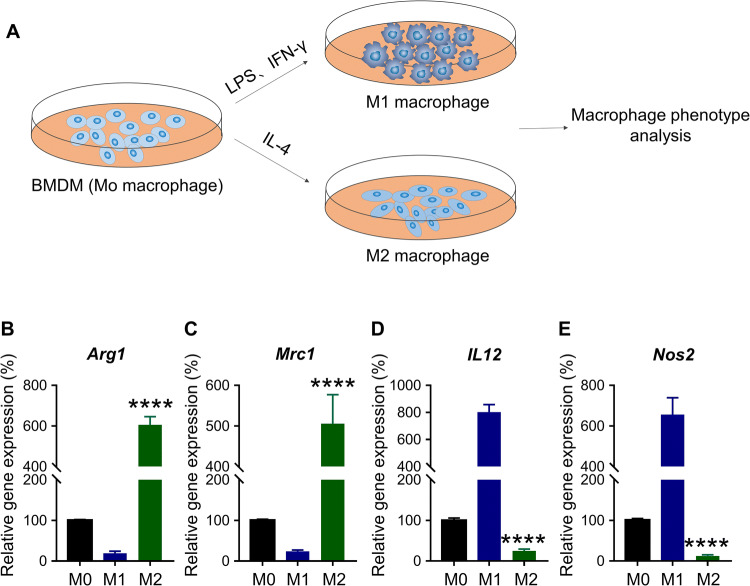
Murine BMDMs at M0 stage were used to generate M1 and M2 macrophages. The isolated BMDMs at M0 state were induced with IFN-γ and LPS for the M1 polarisation, and IL-4 for the M2 polarisation (Fig. 1a) [17]. Cells were induced for 24 h, and transcriptional analysis was conducted using qRT-PCR to assess the polarisation state. ARG1 and MRC1 are characteristic of M2 macrophages, while IL-12 and NOS2 are characteristic of M1 macrophages [17]. It was shown in Fig. 1 that the IL-4-induced macrophages expressed Arg1 and Mrc1 significantly higher than the LPS/IFN-γ induced macrophages (****p < 0.0001) (Fig. 1b, c). In contrast, the former expressed Il12 and Nos2 significantly lower than the later (****p < 0.0001) (Fig. 1d, e). This confirmed that LPS/IFN-γ and IL-4 successfully induced M1 and M2 macrophage polarisation.
Fig. 1.
Macrophage repolarisation assay in vitro. a Schematic representation of macrophage polarisation assay. BMDMs-derived murine macrophages were treated with LPS/IFN-γ or IL-4 for 24 h to induce M1 or M2 polarisation, respectively. The untreated (or undifferentiated) macrophage is regarded as M0 phenotype. b–e Transcription level of Arg1, Mrc1, Il12 and Nos2 in three differently induced macrophages. ****p < 0.0001 (versus M1 macrophage)
Autophagy inhibitor chloroquine (CQ) repolarises M2 macrophages to M1 phenotype in vitro
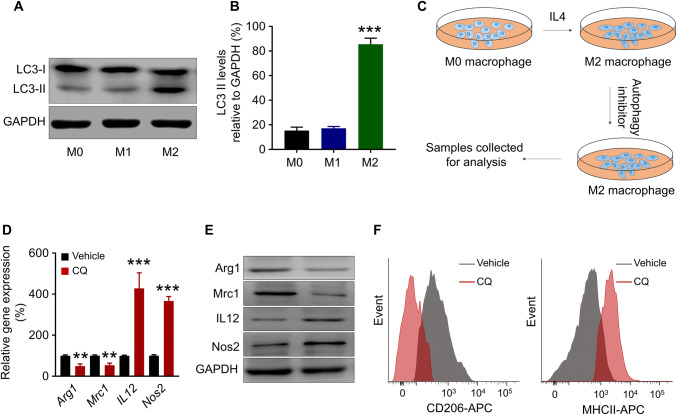
A higher level of autophagic activity was proposed to be a signature of M2 macrophages when compared to M1 macrophages [16]. In this study, this characteristic of M2 macrophages was verified by the analysis of the microtubule-associated protein 1 light chain 3 (LC3) in the polarised M1 and M2 macrophages. LC3 associates with the membrane of the autophagosome and will be converted from a soluble form (LC3-I) to a lipidated form (LC3-II) during the autophagy process [20]. Hence, LC3-II level is positively correlated with the autophagic activity in a cell. The LC3-II level in different macrophage states was examined using western blot (Fig. 2a), and densitometric analysis demonstrated that M2 macrophages contained significantly higher level of LC3-II than M1 macrophages (***p < 0.001) (Fig. 2b), indicating that M2 macrophages do have higher level of autophagic activity.
Fig. 2.
Autophagy inhibition repolarises M2 macrophage towards M1 phenotype. a Western blot and b densitometric analysis of LC3-II levels in M1 and M2 macrophages. GAPDH was used as a loading control. ***p < 0.001 (M2 versus M1 macrophage). c Schematic representation of macrophage repolarisation assay. d Transcription level of Arg1, Mrc1, Il12 and Nos2 in M2 macrophages after CQ or vehicle treatment. **p < 0.005, ***p < 0.001 (versus vehicle-treated M2 macrophages). e Western blot of ARG1, MRC1, IL12 and NOS2 in M2 macrophages after CQ or vehicle treatment. f Flow cytometry demonstrating expression of CD206 and MHCII on the M2 macrophage at the 24 h time point following CQ or vehicle treatment
To test whether autophagy inhibition could repolarise M2 macrophages to M1 phenotype, the polarised M2 macrophages were treated with 20 μM CQ, an autophagy inhibitor, for 24 h (Fig. 2c). The qRT-PCR analysis revealed that after CQ treatment, the transcription of M2 characteristic genes Arg1 and Mrc1 was down-regulated (**p < 0.005), while the transcription of M1 characteristic genes Il12 and Nos2 was up-regulated (***p < 0.001) (Fig. 2d). The changes of the protein level of ARG1, MRC1, IL12 and NOS2 agreed with the transcriptional changes as well (Fig. 2e). This result indicated that CQ induced an M2-to-M1 repolarisation. To further confirm the repolarisation of the macrophages, fluorescence-activated cell sorting (FACS) was used to analyse the resultant cell populations for CD206 (M2 marker) and MHCII (M1 marker), respectively. It was demonstrated that compared to the control group, the CQ-treated M2 macrophages expressed less CD206 but more MHCII, indicating a skew to M1 phenotype (Fig. 2f). These results suggested that CQ could be an M2-to-M1 repolarising agent.
M2-to-M1 repolarisation increases phagocytotic tumour cell killing and restores cisplatin (CDDP) sensitivity of tumour cells
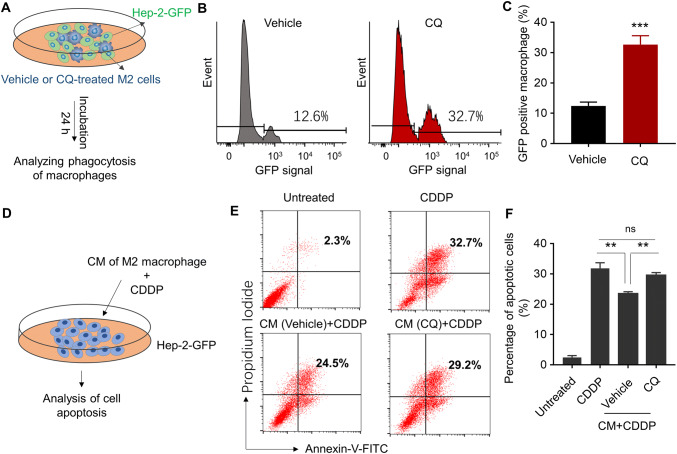
Given the different roles played by M1 and M2 macrophages in the tumour microenvironment, it was proposed that repolarising M2 macrophages to M1 phenotype could enhance their anti-tumour functions [16, 21]. Herein, human laryngeal cancer cell Hep-2 cell line was used to test the anti-tumour ability of the repolarised macrophages. It was first assessed whether M2-to-M1 repolarisation enhances direct tumour cell killing by phagocytosis. M2 macrophages were pre-treated with CQ for M2-to-M1 repolarisation and the repolarised macrophages were co-cultured with GFP-labelled Hep-2 cells for 24 h (Fig. 3a). Flow cytometry was used to detect the GFP signal concurrent with F4/80 pan-macrophage selection marker. The concurrence of the signals will indicate the phagocytosis of Hep-2-GFP cell by macrophages [22]. It was demonstrated that the occurrence of GFP was significantly more frequent for the CQ-treated M2 macrophages than the vehicle-treated M2 macrophages (Fig. 3b, c), suggesting that the M2-to-M1 repolarisation led to more active phagocytosis towards the tumour cells.
Fig. 3.
Repolarisation of M2 macrophage enhances the phagocytosis of macrophages and increases sensitivity of human laryngeal cancer cells to cisplatin (CDDP). a Schematic representation of phagocytosis assay. GFP-labelled Hep-2 cells (Hep-2-GFP) were co-cultured with pre-treated M2 macrophage. The macrophage phagocytosis of tumour cells was examined 24 h post-incubation. b Flow cytometry demonstrating the signal intensity of GFP in the F4/80 positive macrophage. c Quantitative analysis of GFP positive macrophage after incubation. ***p < 0.001 (versus vehicle-treated group). d Schematic representation of drug sensitivity assay. The M2 macrophages were pre-treated with vehicle or CQ (20 μM) for 24 h; the conditioned medium (CM) was collected. Hep-2 cells were incubated with conditioned medium (CM) and then treated with cisplatin (CDDP, 2.5 μM) for 48 h. Cell apoptosis was examined by annexin V/propidium iodide apoptosis assay. e Flow cytometry demonstrating the apoptotic cells. f Quantitative analysis of apoptotic cells. **p < 0.005. ns no significant difference
Besides the phagocytotic killing of tumour cells, it was also assessed whether M2-to-M1 repolarisation could increase chemotherapeutic efficacy by the release of mediators. The CQ-treated M2 macrophages and the vehicle-treated M2 macrophages were pre-cultured for 24 h, and the conditioned media were collected to grow the Hep-2 tumour cells (Fig. 3d). CDDP, a chemotherapeutic agent, was added to the medium, and the apoptosis of Hep-2 cells was measured after 48 h incubation. Annexin V/propidium iodide apoptosis assay demonstrated that the M2 macrophage (vehicle-treated M2) conditioned media significantly reduced apoptosis of Hep-2 cells when compared to the non-conditioned media (Fig. 3e, f). It suggested that M2 macrophages secreted mediators to de-sensitise Hep-2 cells to CDDP. However, the repolarised macrophage (CQ-treated M2) conditioned media significantly increased the apoptosis of Hep-2 cells again (Fig. 3e, f). The apoptosis of Hep-2 cells was unlikely attributed to the tumoricidal effect of CQ in the media, as a control experiment showed that CQ treatment had no impact on the viability of Hep-2 cells in vitro (Fig. S1). Additionally, the effect of the CQ-treated M2 macrophages conditioned media was comparable to the M1 macrophages conditioned media (Fig. S2). It seemed that although M2 macrophages reduce CDDP sensitivity of Hep-2 cells, M2-to-M1 repolarisation could reverse this effect and this process is mediated by the molecules released by the macrophages to the environment.
Anti-tumour activity of CDDP, CQ and CQ/CDDP combined therapy in vivo
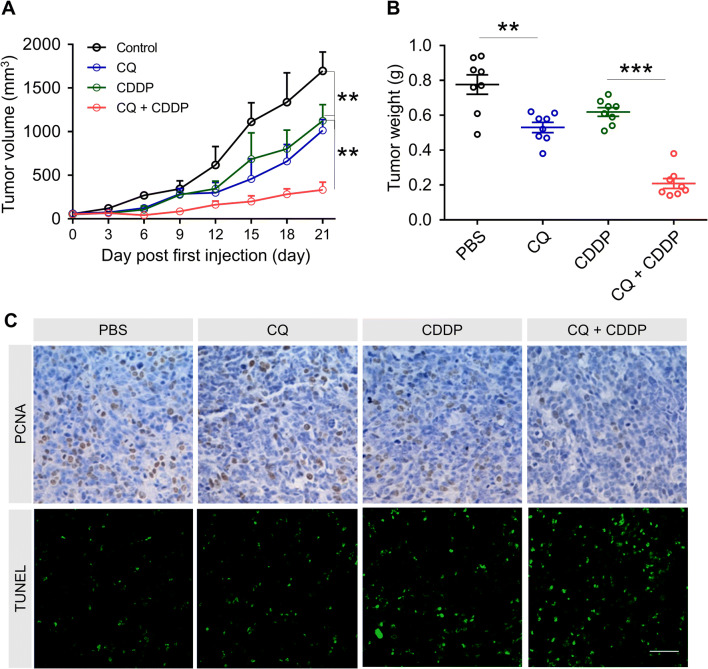
The therapeutic potential of CQ was investigated in vivo using a mouse model of human laryngeal cancer Hep-2 cells. The Hep-2 cells were subcutaneously injected under the skin of the right back of the nu/nu mice. After the sizes of the tumours reached 50 mm3, CDDP, CQ, CDDP + CQ or the control treatment was given every 3 days for five times. It was observed that CQ alone significantly suppressed the tumour volume and the tumour weight growth compared to the control group. The CQ/CDDP combined treatment had a significantly better tumour suppression effect compared to the CDDP group as well (Fig. 4a, b). PCNA analysis and TUNEL analysis were conducted to dissect the mechanism of the anti-tumour effects (Fig. 4c). Quantitative analysis showed that there were less PCNA-positive cells in the CQ/CDDP combined treatment group, indicating a more effective cell proliferation inhibition (Fig. S3A). Meanwhile, there were significantly more TUNEL-positive cells in the CQ/CDDP treatment group, indicating an enhanced cell apoptosis (Fig. S3B). These data suggested the therapeutic potential of CQ, as it inhibited tumour growth and improved CDDP efficacy.
Fig. 4.
In vivo anti-tumour effect of CQ, CDDP and combined treatment. a Tumour growth curves by various treatments in Hep-2 tumour-bearing mice (n = 8). **p < 0.005 (CQ group versus PBS group, CQ + CDDP group versus CDDP group). b Tumour weights of different group at the end of treatments. **p < 0.005 (CQ group versus PBS group), ***p < 0.001 (CQ + CDDP group versus CDDP group). c PCNA analysis and TUNEL analysis of tumour tissues after treatment. The PCNA-positive proliferating cells are stained brown; TUNEL-positive cells are green. Scale bar was 20 μm
CQ treatment inhibits autophagy and repolarises TAMs to M1 phenotype in Hep-2 tumours
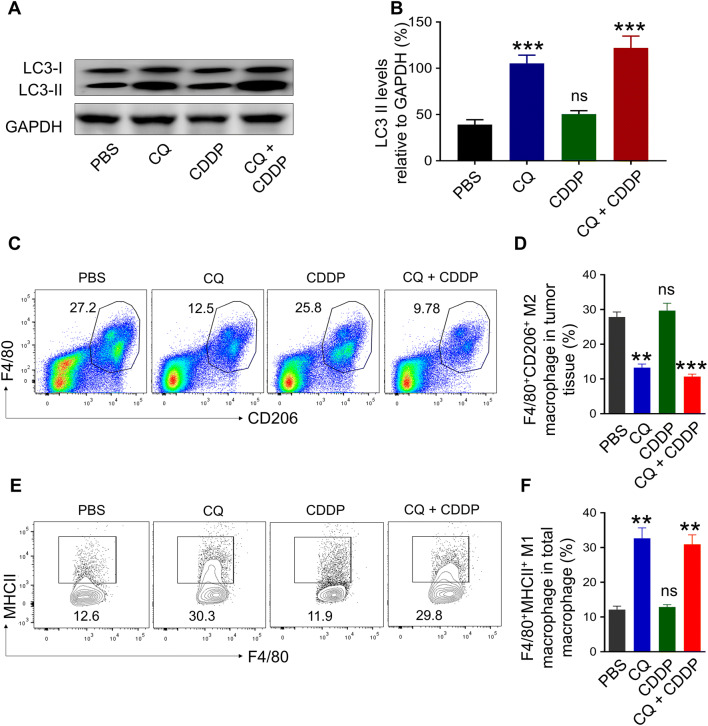
To understand how CQ affected the tumour growth in vivo, it was then assessed whether CQ altered the autophagy level and repolarised TAMs in the tumour tissue. Figure 5b illustrates that the level of LC3-II significantly increased in the CQ-treated group (***p < 0.001) and the CQ/CDDP combined therapy-treated group (***p < 0.001). CQ blocks lysosome acidification and autophagosome degradation, which may lead to the LC3-II accumulation [20]. Thus, the elevated level of LC3-II indicated a generally impeded autophagy process in the tumour tissue. As for the TAMs, flow cytometry was used to detect the CD11b+F4/80+CD206+ M2 macrophages and CD11b+F4/80+MHCII+ M1 macrophages. It was revealed that CQ treatment significantly reduced the percentage of M2 macrophages in the cell population by more than 50% (CQ: **p < 0.005; CQ + CDDP: ***p < 0.001) (Fig. 5c, d). Meanwhile, it increased the percentage of M1 macrophages by more than 200% (CQ: **p < 0.005; CQ + CDDP: **p < 0.005) (Fig. 5e, f). Taking together, these data indicated that CQ reduced the autophagy in the tumour tissue and repolarised M2 macrophages to create an M1-dominated TAM population.
Fig. 5.
Combination therapy inhibits autophagy and repolarises M2 macrophage in vivo. a Western blot and b densitometric analysis of LC3-II levels in tumour tissues after CQ, CDDP or CQ/CDDP combined treatment. GAPDH was used as a loading control. c The ratio of CD11b+F4/80+CD206+ M2 macrophages in tumour tissues at the end of treatment. d Bar graph showing the percentage of M2 macrophages in tumour tissues. e The ratio of CD11b+F4/80+MHCII+ M1 macrophages in tumour tissues at the end of treatment. f Bar graph showing the percentage of M1 macrophages in tumour tissues. Data are shown as mean ± SD (n = 8). **p < 0.005, ***p < 0.001. ns no significant difference (versus PBS group)
Discussion
TAMs are key components in the tumour microenvironment. The two subtypes of TAMs, M1 and M2, are tumour-suppressive and tumour-promotive roles, respectively [2, 8]. M2 macrophages are the dominant subtype of the TAMs in a broad range of cancers, including cervical cancer, colorectal cancer, pancreatic cancer, breast cancer, laryngeal cancer, liver cancer, etc. There is abundant evidence showing that M2 macrophages inhibit cytotoxic T cells, promote tumour metastasis and help tumour regrowth after chemotherapeutic treatment [7, 8, 23–26]. Hence, the repolarisation of TAMs from M2 to M1 phenotype has been proposed long before as a cancer treatment strategy. Previously, M2 marker antagonists and plant products have been developed as the TAM repolarising agents [22, 27, 28].
Considering that M2 macrophages rely on autophagic metabolism more than M1 macrophages [16], autophagy inhibitors might be a category of compounds that can be used as the TAM repolarising agents. Here, for the first time, we identified that CQ, an autophagy inhibitor, is able to repolarise M2 macrophages to M1 phenotype both in vitro and in vivo. In the Hep-2 laryngeal tumour model in mice, CQ treatment converted the M2-dominated TAM population to an M1-dominated TAM population. LC3-II expression analysis demonstrated that the TAM repolarisation in vivo was associated with a significant reduction of the autophagy level in the tumour tissue. These results indicate a correlation between autophagy inhibition and TAM repolarisation, suggesting that the TAM repolarising agents could be developed from autophagy inhibitors.
Beside the ability of repolarising TAMs, CQ also showed therapeutic potential in the laryngeal cancer treatment. The CQ/CDDP combined therapy was more effective in Hep-2 tumour growth inhibition than the CDDP treatment alone, and the combined therapy demonstrated significantly enhanced cell apoptosis and reduced cell proliferation. The repolarisation of TAMs induced by CQ might have played an important role in this process, since it was proved in this study that the M2-to-M1 repolarisation would enhance the phagocytotic activity of macrophages towards Hep-2 tumour cells. It was also proved that M2 macrophages secreted mediators to de-sensitise Hep-2 cells to CDDP, but this effect of M2 macrophages could be reversed by M2-to-M2 repolarisation using CQ. This finding is consistent with the proposed tumour-promoting mechanism of TAMs that the M2 macrophages secrete regulatory cytokines to prevent tumour cell apoptosis and promote cancer stem cell proliferation [5, 29]. Therefore, the tumour inhibitory and CDDP enhancing effect of CQ might be achieved through the repolarisation of TAMs.
CQ might have enhanced CDDP treatment outcomes via other mechanisms. It is interesting that although CQ did not demonstrate cytotoxicity against Hep-2 cells in vitro, CQ treatment significantly reduced tumour growth in vivo. Besides TAM repolarisation, another possible explanation is that the hypoxic, nutrition-deficient tumour microenvironment makes Hep-2 cells rely more on the autophagic metabolism. Thus, the inhibition of autophagy might also limit tumour growth under this condition. This proposed mechanism was validated in the pancreatic cancer [20]. The pancreatic cancer cells have a high basal autophagy level even if cultured in media, and autophagy inhibition directly reduced tumour cell proliferation in vitro [20]. It has also been proposed that autophagy enables chemotherapy tolerance by reducing cyclin D1 in the cell and delaying cell cycle to help the tumour cells avoid apoptosis [29]. It has been proved that autophagy inhibition directly abolishes apoptosis evasion of prostate cancer cells in vitro [30]. Hence, CQ might have directly reduced CDDP tolerance of Hep-2 cells through this mechanism. Moreover, it was illustrated by Guo et al. that M2 macrophages would secrete IL-17 to stimulate chaperon-mediated autophagy in tumour cells to help them avoid apoptosis [29]. Together, in this study, the effect of CQ on tumour inhibition and CDDP enhancement might involve multiple mechanisms, including the TAM repolarisation, autophagy inhibition in the tumour cells, re-sensitisation of the tumour cells to CDDP, and the mutual amplification of the above functions.
In this study, a positive influence of autophagy inhibition and TAM repolarisation on Hep-2 laryngeal tumour treatment was demonstrated. Whereas, it is still contextual dependent whether this strategy should be applied for different cancer types and at different cancer developmental stages. First, the correlation between TAMs infiltration and overall survival rate is non-consistent among different types of cancers and different stages of cancer development [8]. For many cancer types, TAMs are populated with M2 macrophages, and the M2 macrophages are considered responsible for the tumour development [7, 8, 23–26]. However, exceptions have been observed. The major subtype of the TAMs might be the anti-tumour M1 macrophages instead of M2 [31]. The composition of TAMs might change between tumour developmental stages [32]. Meanwhile, M1 macrophages might contribute to tumour malignancy as well [33]. Second, it has been suggested that autophagy inhibition plays different roles across tumour developmental stages. On one hand, established tumours rely on autophagy to generate energy and resist chemotherapeutics [20, 29, 30, 34]. On the other hand, there is evidence showing that a normal autophagy level prevents spontaneous tumorigenesis and early development, especially for liver tumours [14]. Additionally, some chemotherapeutic agents kill the tumour cells through the autophagic apoptosis activation [35, 36]. Moreover, autophagy level and TAMs both regulate cytotoxic T cell activity [37], adding further complexity to the cell interaction network. To sum up, TAMs and autophagy are both complicatedly intertwined with tumorigenesis, tumour progression and chemotherapeutic drug sensitivity. Comprehensive assessment will be required to determine when and where to use the autophagy inhibitors and the TAM repolarisation strategy.
Conclusion
In conclusion, TAMs usually emerge as the tumour-promoting M2-subtype in many cancers. We demonstrated that CQ, an autophagy inhibitor, could repolarise TAMs from M2 to the anti-tumour M1 phenotype. CQ treatment reduced Hep-2 laryngeal tumour growth and greatly improved the CDDP treatment outcome in the mouse model. This result might be attributed to the CQ-induced TAM repolarisation and direct autophagy inhibition in the tumour cells. Further tests are required before translating these findings to clinical practice. It needs to be investigated whether this strategy is applicable to other cancer types and different cancer stages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ARG1
Arginase-1
- ATCC
American type culture collection
- BMDM
Bone marrow-derived macrophage
- CDDP
Cisplatin
- CM
Conditioned media
- CQ
Chloroquine
- DMEM
Dulbecco’s modified Eagle medium
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IFN
Interferon
- LC3
Microtubule-associated protein 1 light chain 3
- IL
Interleukin
- LPS
Lipopolysaccharide
- M-CSF
Recombinant human macrophage colony stimulating factor
- MRC1
Mannose receptor-1
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NOS
Nitric oxide synthase
- PBS
Phosphate buffer saline
- qRT-PCR
Quantitative real-time polymerase chain reaction
- TAM
Tumour-associated macrophage
Author contributions
YG, YF, XC, QW conducted the experiments, YG and YF analysed the data. YG and XP wrote the manuscript. XP conceived the study.
Funding
This study was funded by the Project of Medical and Health Technology Development Program in Shandong (No. 2017WSB04024).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Animal studies on C57BL/6 mice and nu/nu nude mice complied with the Guidelines for Animal Studies in Shandong University. Ethical Committee in Shandong Provincial Qianfoshan Hospital reviewed and approved this study (#SDQFSH-2017048).
Animal source
C57BL/6 mice and nu/nu nude mice were purchased from Nanjing Model Animal Institute (Nanjing, China).
Cell line authentication
Human laryngeal cancer Hep-2 cell line and GFP-labelled Hep-2-GFP cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cells were verified by A Short Tandem Repeat analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Cao XT. The origin and function of tumor-associated macrophages. Cell Mol Immunol. 2015;12:4. doi: 10.1038/cmi.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni Y-H, Ding L, Huang X-F, Dong Y-c, Hu Q-G, Hou Y-Y. Microlocalization of CD68(+) tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumor Biol. 2015;36:5291–5298. doi: 10.1007/s13277-015-3189-5. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, Hewitt S, Udupi GM, Gallagher WM, Wegner C, West BL, Wang-Gillam A, Goedegebuure P, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Can Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.Can-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakasone ES, Askautrud HA, Kees T, Park J-H, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan Y-X, Qiu J, Park J, Sinha P, Bissell MJ, Frengen E, Werb Z, Egeblad M. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J-Y, Li X-Y, Tadashi N, Dong P. Clinical significance of tumor-associated macrophage infiltration in supraglottic laryngeal carcinoma. Chin J Cancer. 2011;30:280–286. doi: 10.5732/cjc.010.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Snuderl M, Jain RK. Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity. Cancer Cell. 2011;19:1–2. doi: 10.1016/j.ccr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 11.Colegio OR, Ngoc-Quynh C, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 13.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death differ. 2005;12:1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019;19:170–183. doi: 10.1038/s41577-018-0095-2. [DOI] [PubMed] [Google Scholar]
- 17.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Ahmed RLMS, Kohler RH, Pittet MJ, Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y, Wang Y, Wharen RE, Yun K, Bu G, Knutson KL, Kim BYS. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12:763. doi: 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- 19.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’Antonio G, Mautner J, Tonon G, Haigis M, Shirihai OS, Doglioni C, Bardeesy N, Kimmelman AC. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni A, Chandrasekar V, Natarajan SK, Ramesh A, Pandey P, Nirgud J, Bhatnagar H, Ashok D, Ajay AK, Sengupta S. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng. 2018;2:589. doi: 10.1038/s41551-018-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Zhou R, Wang H, Li W, Pan M, Yao X, Zhan W, Yang S, Xu L, Ding Y, Zhao L. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019 doi: 10.1038/s41418-019-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, Shinchi H, Natsugoe S. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas. 2013;42:155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, Huang Y, Qiu Q, Lin J, Huang X, Tan W, Min C, Wang C. TNF-alpha derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/beta-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 2019 doi: 10.1016/j.yexcr.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Lepique AP, Perez Daghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. 2009;15:4391–4400. doi: 10.1158/1078-0432.Ccr-09-0489. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Bi L, Luo H, Jiang Y, Chen F, Wang Y, Wei G, Chen W. Water extract of ginseng and astragalus regulates macrophage polarization and synergistically enhances DDP’s anticancer effect. J Ethnopharmacol. 2019;232:11–20. doi: 10.1016/j.jep.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo B, Li L, Guo J, Liu A, Wu J, Wang H, Shi J, Pang D, Cao Q. M2 tumor-associated macrophages produce interleukin-17 to suppress oxaliplatin-induced apoptosis in hepatocellular carcinoma. Oncotarget. 2017;8:44465–44476. doi: 10.18632/oncotarget.17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degtyarev M, De Maziere A, Orr C, Lin J, Lee BB, Tien JY, Prior WW, van Dijk S, Wu H, Gray DC, Davis DP, Stern HM, Murray LJ, Hoeflich KP, Klumperman J, Friedman LS, Lin K. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33:118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 32.Perdiguero EG, Geissmann F. Identifying the infiltrators. Science. 2014;344:801–802. doi: 10.1126/science.1255117. [DOI] [PubMed] [Google Scholar]
- 33.Xiao M, Zhang J, Chen W, Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2018 doi: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Investig. 2007;117:326–336. doi: 10.1172/jci28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8:2084–2096. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamed HA, Yacoub A, Park MA, Eulitt P, Sarkar D, Dimitriev IP, Chen C-S, Grant S, Curiel DT, Fisher PB, Dent P. OSU-03012 enhances Ad.mda-7-induced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins. Cancer Biol Ther. 2010;9:526–536. doi: 10.4161/cbt.9.7.11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: a troika governing cancer and its treatment. Cell. 2016;166:288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.