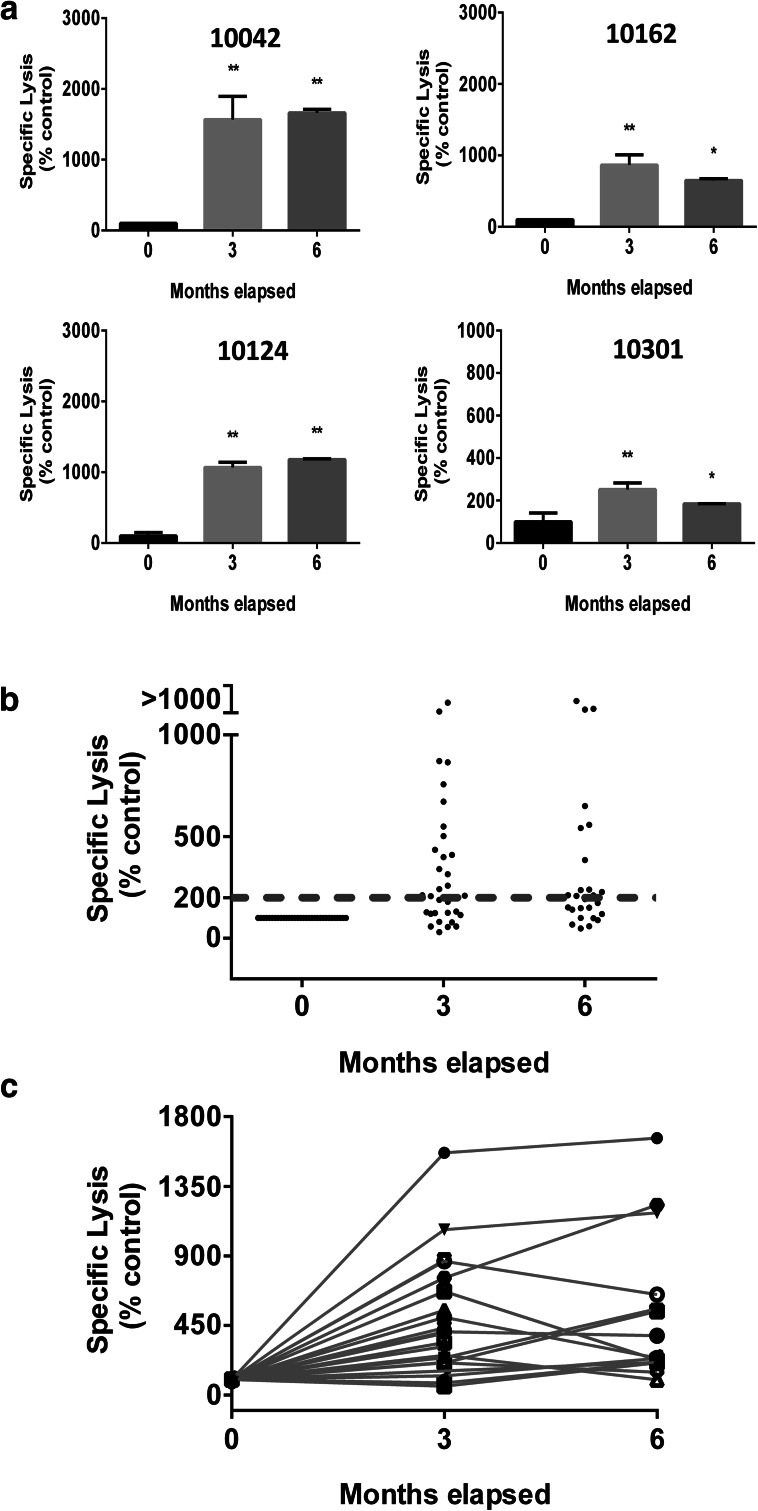
Fig. 3.
ADCC induced by anti-NeuGcGM3 Ab present in serum samples from racotumomab-vaccinated patients. Specific lysis against X63 cells was analyzed using a LDH-release assay and effector cells from healthy donors. a ADCC activity (indicated as specific lysis) obtained with hyperimmune serum samples corresponding to the third and the sixth months post-vaccination from four representative patients. Bars indicate mean values ± standard deviation. Patients’ identification numbers are indicated on each graph. ANOVA followed by Dunnett’s multiple comparison test was used to analyze differences between groups; *p < 0.05, **p < 0.01. b ADCC response obtained for 36 racotumomab vaccinated NSCLC patients using basal and hyperimmune serum samples. Results are expressed as percentage respect to basal samples (0 month). Dotted line indicates the minimum specific lysis value to be considered a positive ADCC response (threshold value). Kruskal–Wallis followed by Dunnett’s multiple comparison test was used to analyze differences between groups; p < 0.001 basal vs month 3 and basal vs month 6. c ADCC activity developed by anti-NeuGcGM3 Ab present in basal and hyperimmune samples