Abstract
Adoptive cell therapy with tumor-infiltrating lymphocytes (TIL) can mediate objective responses in up to 50% of malignant melanoma patients with a good performance status refractory to standard treatments. Current protocols for generation of TILs rely on open surgery for access to tumor tissue. We obtained tumor material by ultrasound-guided core needle biopsy or surgery from melanoma patients with progressive disease and were able to isolate >5 × 106 TILs from 23 of 24 patients who were subsequently treated with these cells. One-third of the individual TIL-positive cultures displayed interferon gamma activity after stimulation with relevant melanoma cell lines. When expanded TILs were used for treatment in combination with daily low dose s.c. IL-2 after prior lymphodepleting chemotherapy, we observed objective clinical responses in one patient treated with TILs obtained from surgery and 4 patients treated with TILs from core biopsies. The results of this study demonstrate for the first time the potential of core biopsies for generation of relevant numbers of TILs that can mediate objective responses in patients with metastatic malignant melanoma. Ultrasound-guided core needle biopsy is a robust, safe and inexpensive approach to obtain tumor tissue for TIL generation, and is especially valuable in instances where surgery is contraindicated.
Keywords: Melanoma, Tumor-infiltrating lymphocyte, Adoptive cell therapy, Core needle biopsy
Introduction
Melanoma is one of the most common causes of cancer-related mortality among young adults in northern Europe and shows an increasing incidence compared with other cancers. The tumor is widely considered immunogenic with the ability to elicit immune responses that may result in the accumulation of tumor-infiltrating lymphocytes (TIL) [1]. Adoptive cell therapy (ACT) with autologous TILs isolated from melanoma biopsies was developed at the National Cancer Institute (NCI, Bethesda, MD), and represents a promising approach for treatment of patients in a good performance status with refractory metastatic disease [2]. Tumor biopsies are obtained through surgical metastasectomy and are processed in the laboratory to generate TIL cultures from tumor fragments or digests [3]. Dudley et al. [4] reported objective responses according to the Response Evaluation Criteria in Solid Tumors (RECIST) in 18 out of 35 patients (51%), with durable and ongoing responses beyond several years. Similar response rates (50%) were confirmed in a later series of 20 patients [5]. After addition of whole body irradiation, even higher response rates may be achieved, but this needs to be confirmed. In a preclinical study, we reported the identification of melanoma-reactive TIL cultures in tumor biopsies of 25 of 30 (83%) melanoma patients. Of these, 29% contained IFNγ-producing cells after in vitro stimulation with HLA semi-matched melanoma cell lines. We reported on successful isolation of TILs from 15 core biopsies [6] and 18 surgically removed tissues [7]. In the current study, our main aim was large-scale expansion of TILs to clinically relevant numbers for subsequent treatment of 24 patients. The rapid expansion protocol (REP) from NIH [3] was used to expand TILs isolated from core biopsies (N = 11) or surgical metastasectomy (N = 13). The high dose i.v. IL-2 used in earlier clinical studies has sometimes been paralleled with severe side effects. Therefore, we sought to refine earlier applied treatment protocols by using low-dose s.c. instead of high-dose i.v. IL-2. When expanded TILs were used for treatment in combination with daily low-dose IL-2 and after lymphodepleting chemotherapy, we observed objective clinical responses (OR) in 1 patient (OR = 7%) treated with TILs obtained from surgery and 4 patients (OR = 36%) treated with TILs from core biopsies. The results of this study demonstrate for the first time the capacity of core biopsies to generate relevant numbers of TILs that mediate objective responses in patients with metastatic malignant melanoma.
Materials and methods
Patient characteristics
From June 2005 to January 2010, we treated 28 patients with stage IV malignant melanoma with autologous TILs. Four of these patients were not included in the current study since they did not receive chemotherapy or IL-2. The average age of the 24 patients was 53 years. All patients had measurable disease on CT and MRT scans and were with one exception (#13) refractory to standard treatments including surgery, several lines of chemotherapy, immunotherapy including s.c. IL-2 and radiotherapy. The patients were recruited and treated at Uppsala University Hospital. Cutaneous lesions were the most prominent initial tumor sites (18/24). Tumor was resected as part of an Ethical Committee approved (reference number 2005:383) clinical study. Tumor tissue was removed by ultrasound-guided core needle biopsy according to hospital standards [6]. Biopsies were obtained with 18 gauge needles and were on average 2 cm long and 0.16 cm in diameter. Three to four separate needle insertions were typically performed, and all biopsies were histologically confirmed as melanoma when appropriate. The HLA-A types for all patients have been assessed [7] and HLA-A2 status as well as other back ground data is presented in Table 1.
Table 1.
Background information
| Patient characteristics | Tumor characteristics | Previous treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat. no. | Sex | Agea | Performance status (WHO) | HLA A2+ | Recovery of tumor material | Initial tumor site | Biopsy site | Tumor sites upon treatment | Chemob | Immuno | Radio |
| 1 | M | 58 | 2 | Y | S | Ocular | SC | Li, Lu, SC, LN, Pa, Mes | 2 | IL-2 | Y |
| 2 | M | 49 | 1 | Y | S | Skin | SC | SC, LN | 2 | IFN | Y |
| 3 | M | 56 | 1 | N | S | Skin | Neck | Li, Lu, LN, SC, Ki | 2 | αCTLA-4 | N |
| 4 | M | 66 | 0 | N | S | Skin | Neck | Lu, He, SC | 1 | – | N |
| 5 | F | 55 | 3 | Y | S | Skin | Groin | LN | 1 | – | Y |
| 6 | M | 39 | 1 | N | S | Skin | Abdomen | Li, SC, Br | 3 | Sorafenib | N |
| 7 | F | 65 | 2 | N | S | Unknown | SC | Li, Lu, SC, Br | 2 | – | N |
| 8 | M | 70 | 0 | N | S | Unknown | Rib | Sk, Ki, Li, muscle | 1 | IFN | N |
| 9 | M | 73 | 0 | Y | S | Skin | LN axilla | LN, Li, Lu | 1 | – | N |
| 10 | F | 45 | 1 | Y | S | Skin | SC | Breast | 3 | Bevacizumab | N |
| 11 | F | 17 | 0 | N | S | Skin | SC | SC | 1 | IFN | Y |
| 12 | M | 46 | 0 | N | S | Skin | SC | Li, Lu, LN, SC | 3 | IL-2, Bevacizumab | Y |
| 13 | M | 66 | 0 | Y | S | Skin | SC | SC, Lu, Med | 0 | – | N |
| 14 | F | 44 | 2 | Y | NB | Skin | Liver | Li, Lu, SC, LN, Ki | 2 | – | Y |
| 15 | F | 58 | 0 | Y | NB | Skin | Lung | Lu, Mes | 1 | IFN | Y |
| 16 | F | 43 | 1 | N | NB | Skin | Spleen | Lu, LN, Sp | 2 | – | N |
| 17 | M | 60 | 0 | N | NB | Unknown | Neck | Sk, Thy | 1 | IFN | Y |
| 18 | F | 65 | 0 | N | NB | Skin | Abdomen | Li, Lu, Br, LN, Ki | 2 | IFN, αCTLA-4 | Y |
| 19 | F | 32 | 0 | Y | NB | Skin | Liver | Li, Lu, CNS, LN | 1 | – | Y |
| 20 | M | 62 | 1 | N | NB | Skin | Liver | Lu, Li, Sp | 1 | – | Y |
| 21 | M | 58 | 0 | N | NB | Skin | Liver | Li, Lu | 2 | – | N |
| 22 | M | 55 | 2 | Y | NB | Skin | Abdomen | Li, LN, SC, Sm In, GB | 2 | IL-2 | Y |
| 23 | M | 29 | 1 | N | NB | Ocular | Liver | Li, Sk | 1 | – | Y |
| 24 | F | 51 | 1 | Y | NB | Ocular | Pelvis | SC, Mes, Sp, Pa, LN | 2 | IFN | Y |
aAge at the initiation of treatment, bNumber of lines of chemotherapy
Anatomical abbreviations: Li liver, Lu lung, LN lymph node, SC subcutaneous, Pa pancreas, Mes mesenterium, Ki kidney, Med mediastinum, Sk skeleton, Sp spleen, He heart, Thy thyroid, Br brain, Sm In small intestine, GB gall bladder
Other abbreviations: S surgery, NB needle biopsy
Isolation and characterization of TIL
TILs were prepared as previously described [3]. Briefly, core needle biopsies were cut to 0.1-cm-long pieces and suspended in 2 ml complete medium and IL-2 (6,000 IU/ml, Novartis, Basel, Switzerland) in a humidified 37°C incubator with 5% CO2. Complete media (CM) consisted of RPMI-1640 supplemented with 10% pooled human AB serum, 1% PEST, 1% HEPES, 0.5% l-Glutamine and 0.2% 2-mercaptoethanol. All cell culture reagents were purchased from Invitrogen, Carlsbad, CA. 5 to 7 days after initiation, 1 ml of the media replaced with fresh CM and IL-2. Media was replaced every 2–3 days thereafter and confluent wells were split into larger wells in order to maintain a cell density of 1–1.5 × 106 cells/ml. Eleven to 24 individual cultures were established for each patient and only cultures with cell numbers >5 × 106 were considered positive for TIL growth.
IFNγ production by intracellular staining was performed as described [8]. In short, 7.5 × 105 TILs were co-incubated at a 1:1 ratio with patient HLA semi-matched or HLA unmatched melanoma cell lines. Brefeldin A (Sigma-Aldrich, St. Louis, MO) was added to block secretion and the cells were permeabilized, stained with conjugated antibodies (CD3, CD8 and IFNγ from BD Biosciences) and analyzed by FACS. All cultures displaying >0.1% IFNγ activity were considered as positive. IFNγ production was never detected after stimulation with HLA unmatched melanoma cell lines.
Rapid expansion of TILs
Rapid expansions of TILs were performed using the Rapid Expansion Protocol [3, 9]. Briefly, TILs were cultured in standing T flasks with 200-fold excess of irradiated (55 Gy) allogeneic blood mononuclear cells from several healthy donors as feeder cells. Cells were cultured in CM with 5% human AB serum, 30 ng/ml agonistic anti-CD3 antibody (Ortho Biotech, Bridgewater, NJ) and 600 IU/ml IL-2. Half the media was changed on day 5 using CM and 600 IU/ml IL-2 and split to larger T flask or gas-permeable bags as needed thereafter.
We observed an average of 200-fold expansion for TILs isolated from surgically obtained tumors and an average of 138-fold expansion for TILs from core biopsies. Final numbers of administered TILs varied based on growth rates of cells. A mean of 9.4 × 109 (range 0.7–30 × 109) TILs from surgery and 5.4 × 109 (range 0.5–20 × 109) from core biopsies were infused (Table 2).
Table 2.
No of tissue samples and characterization of TILs
| Pat. no. | Number of tissue samples | TIL positive cultures | CD8+ CD3+ (%) | IFNγ production | Fold expansion | TILs infused (×109) |
|---|---|---|---|---|---|---|
| 1 | 1 | 23/24 | 53 | 2/23 | 15 | 0.7 |
| 2 | 2 | 22/22 | 80 | 0/22 | 880 | 1.8 |
| 3 | 1 | 16/18 | 41 | 4/16 | 216 | 1.6 |
| 4 | 1 | 20/24 | 93 | 5/20 | 1,100 | 30.0 |
| 5 | 2 | 1/12 | 55 | NA | 146 | 8.9 |
| 6 | 1 | 20/24 | 97 | 9/20 | 500 | 20.0 |
| 7 | 1 | 32/36 | 5 | 4/32 | 200 | 8.0 |
| 8 | 1 | 23/24 | 90 | NA | 250 | 10.0 |
| 9 | 1 | 12/24 | 56 | NA | 500 | 20.0 |
| 10 | 1 | 9/24 | 22 | NA | 150 | 6.0 |
| 11 | 1 | 14/24 | 65 | NA | 180 | 7.2 |
| 12 | 1 | 5/24 | 68 | NA | 125 | 5.0 |
| 13 | 1 | 10/24 | 42 | NA | 89 | 3.5 |
| 14 | 4 | 4/12 | 54 | 4/4 | 45 | 2.0 |
| 15 | 4 | 12/16 | 85 | 7/12 | 77 | 1.6 |
| 16 | 4 | 10/13 | 51 | 4/10 | 230 | 3.0 |
| 17 | 4 | 9/24 | 77 | 5/9 | 278 | 9.7 |
| 18 | 4 | 1/12 | 41 | NA | 512 | 20.0 |
| 19 | 4 | 7/24 | 33 | 1/7 | 277 | 6.0 |
| 20 | 3 | 8/24 | 80 | 3/8 | 15 | 0.6 |
| 21 | 4 | 5/24 | 12 | 0/5 | 125 | 5.0 |
| 22 | 3 | 7/16 | 76 | NA | 132 | 5.3 |
| 23 | 3 | 13/24 | 47 | 2/13 | 138 | 5.5 |
| 24 | 4 | 16/24 | 65 | 2/16 | 50 | 0.5 |
NA not analyzed
Treatment and clinical assessment
Patients received nonmyeloablative lymphodepleting preconditioning regimen consisting of 2 days of cyclophosphamide (60 mg/kg) i.v. followed by 5 days of fludarabine (25 mg/m2) i.v. On the day following the final dose of fludarabine, patients received cell infusion of TILs i.v. and low dose daily s.c. IL-2 therapy (2.4 × 106 units/m2) was initiated and maintained until progressive disease or unacceptable toxicity. Assessment of response was through CT scans at 4 weeks following cell infusion and thereafter when appropriate on an individual basis. Patient responses were categorized by an experienced radiologist in accordance with RECIST and included complete response (CR)—disappearance of all tumor foci; partial response (PR)—a reduction of at least 30% in tumor diameters; stable disease (SD)—neither partial response nor progressive disease; and progressive disease (PD)—at least a 20% increase in the sum of all tumor dimensions from the smallest tumor size or the appearance of a new tumor lesion [10, 11]. The term objective response refers to either CR or PR.
Statistical analysis
One-way ANOVA test was used to determine the association between the number of taken biopsies, TIL growth, and specificity. The Mann–Whitney test was performed to compare the methods for obtaining biopsies in fold expansion and cell numbers after rapid expansion. All P values >0.05 were considered as statistically insignificant. Analysis was performed using GraphPad prism software version 5.01 (La Jolla, CA, USA).
Results
Core biopsy vs. surgery
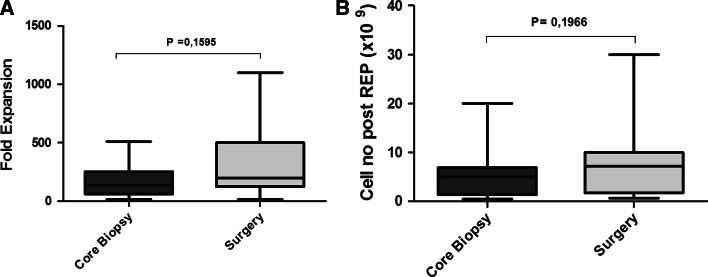
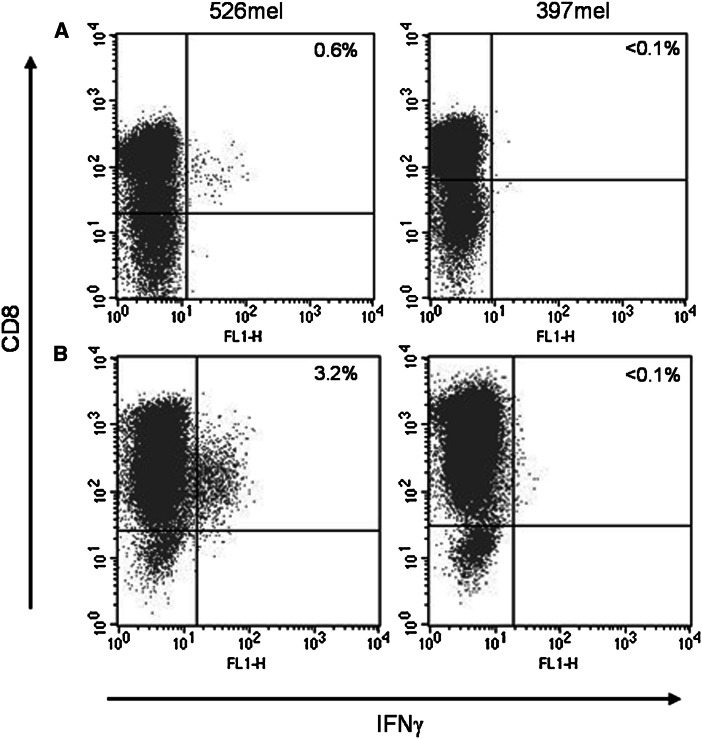
Eight of 11 patients (73%) underwent 4 needle biopsies, while 3 of 11 (27%) underwent 3 biopsies. We received in total 41 core biopsies of which 16 (41%) were pigmented and resembled melanocytic lesions. Totally 209 individual cultures were established and 84 (40%) were positive for active TILs after on average 42 days in culture. We observed cell numbers >5 × 106 in 10 of 11 patients. The proportion of CD8+CD3+ cells was on average 63% (range 12–85%) of the total cell number. Of the 84 individual TIL-positive cultures, 30 (36%) displayed IFNγ activity when stimulated with HLA-matched melanoma cell lines. No activity could be recorded in the negative controls. No correlations were found between the number of needle biopsies taken and cell yield (P = 0.39) or IFNγ activity (P = 0.94). We found no correlation between pigmentation of biopsies and IFNγ activity or the clinical potency of the isolated TILs. The amount of surgically resected tissue varied among individual patients but allowed for isolation of >5 × 106 TILs from all patients. Both methods for obtaining TILs yielded comparable cell numbers after expansion (Fig. 1). The number of CD8+CD3+ isolated from surgical biopsies was on average 58% (range 5–97%) of the total cell number and 25% of the cultures displayed IFNγ activity. A representative FACS plot of IFNγ reactivity by TILs isolated from the biopsies of two patients is shown in Fig. 2. All results are summarized in Table 2.
Fig. 1.
Fold expansion (a) and absolute cell numbers (b) after rapid expansion of TILs isolated from core biopsies (dark gray) or surgically retrieved tissue (light gray). Mann–Whitney testing was used to evaluate differences between the two methods of biopsy for obtaining tumor material
Fig. 2.
TILs obtained from a patient #5 and b patient #19 were cultured from tumor tissue and analyzed for IFNγ production after stimulation with HLA semi-matched or nonmatched melanoma cell lines 526mel and 397mel, respectively. Cultures displaying >0.1% IFNγ activity were considered as positive
In hospital period
Average days (mean) of hospitalization in association with treatment were 23 (range 14–62 days). Fifty percent (12/24) of the patients required transfusion of platelets and 71% (17/24) transfusions of erythrocytes in association with treatment. During treatment, 14 of 24 patients had febrile neutropenia with neutrophil count of <109 for 5 days on average. Individual data for the in hospital period are found in Table 3.
Table 3.
Treatment-related clinical data
| Pat. no. | IL-2 treatment post-ACT | Transfusionsa | Febrile neutropenia | Neutrophils <0.5 (days) | Hospitalization (days)a | Clinical response to treatment | Survival post-treatment (weeks) | ||
|---|---|---|---|---|---|---|---|---|---|
| Toxicity/symptoms | Duration | Platelets (units) | Erythrocytes (units) | ||||||
| 1 | – | 5 w | 6 | 2 | N | 9 | 22 | SD | 8 |
| 2 | Fever, chills | 1 m | 0 | 4 | N | 3 | 24 | SD | 17 |
| 3 | Fever, muscle pain | 1 w | 0 | 2 | N | 6 | 18 | SD | 14 |
| 4 | Local side effects | 2 m | 0 | 0 | N | 4 | 19 | PR | 53 |
| 5 | Fever, muscle pain | 1 w | 10 | 6 | Y | 9 | 62 | SD | 4 |
| 6 | – | 3 w | 1 | 0 | Y | 5 | 20 | PD | 4 |
| 7 | – | 1½ m | 2 | 2 | Y | 7 | 29 | SD | 13 |
| 8 | Fever | 1 m | 1 | 4 | Y | 6 | 18 | SD (−9%) | 40 |
| 9 | – | 3 w | 1 | 2 | Y | 8 | 21 | SD (−16%) | 60 |
| 10 | Chills | 1 d | 0 | 4 | Y | 4 | 27 | SD (−24%) | 18 |
| 11 | – | 5½ m | 0 | 0 | N | 4 | 14 | PD | 142+ |
| 12 | Fever, Chills | 1 w | 7 | 4 | N | 5 | 26 | SD | 17 |
| 13 | Fevera | 4 m | 0 | 0 | Y | 4 | 22 | SD | 16 |
| 14 | Chills | 3 m | 0 | 4 | Missing data | 6 | 24 | SD | 19 |
| 15 | Fever, fatigue | 1 m | 2 | 6 | Y | 7 | 16 | SD (−4%) | 10 |
| 16 | – | 4 m | 4 | 14 | Y | 7 | 26 | PR | 19 |
| 17 | – | Ongoing | 1 | 0 | N | 5 | 21 | CR (09/2011) | 191+ |
| 18 | – | 3 d | 2 | 4 | Y | 4 | 16 | PD | 1/2 |
| 19 | – | 6 m | 0 | 2 | Y | 4 | 21 | PR | 30 |
| 20 | Fever, fluid retention | 3 m | 0 | 0 | N | 4 | 19 | SD (−1%) | 17 |
| 21 | – | 1 m | 0 | 4 | Y | 7 | 20 | SD | 5 |
| 22 | Fevera | 5 m | 3 | 9 | Y | 8 | 28 | PR | 32 |
| 23 | Gastritis | 4 m | 0 | 0 | Y | 4 | 18 | PD | 49 |
| 24 | Local side effects | 5 m | 0 | 2 | N | 5 | 23 | SD (−6%) | 57 |
+ Alive 15/11/2011, Clinical response abbreviations: SD stable disease, CR complete response, PD progressive disease, PR partial response
aIn association with treatment. m months, w weeks, d days
For patients assessed as SD with some tumor regression, this regression according to RECIST criteria is denoted in brackets
Clinical results
Patient clinical outcomes are shown in Table 2. The mean survival time was 35 weeks (range 4 days to 44 months). The patient experiencing from CR (#17) had still no signs of relapse at most recent follow-up according to scans and clinical assessment at 42 months. Patient #19 was assessed as having SD 1 month post-treatment, but at 2 months after the T-cell infusion, a PR was demonstrated. For patient #16, the response was more pronounced at month two as compared to month one after treatment. One patient (#18) died within 1 week after the TIL infusion. Her decease was probably treatment related. Following the chemotherapy, the patient developed severe thrombocytopenia and suffered from brain hemorrhage followed by death 11 days after the TIL infusion.
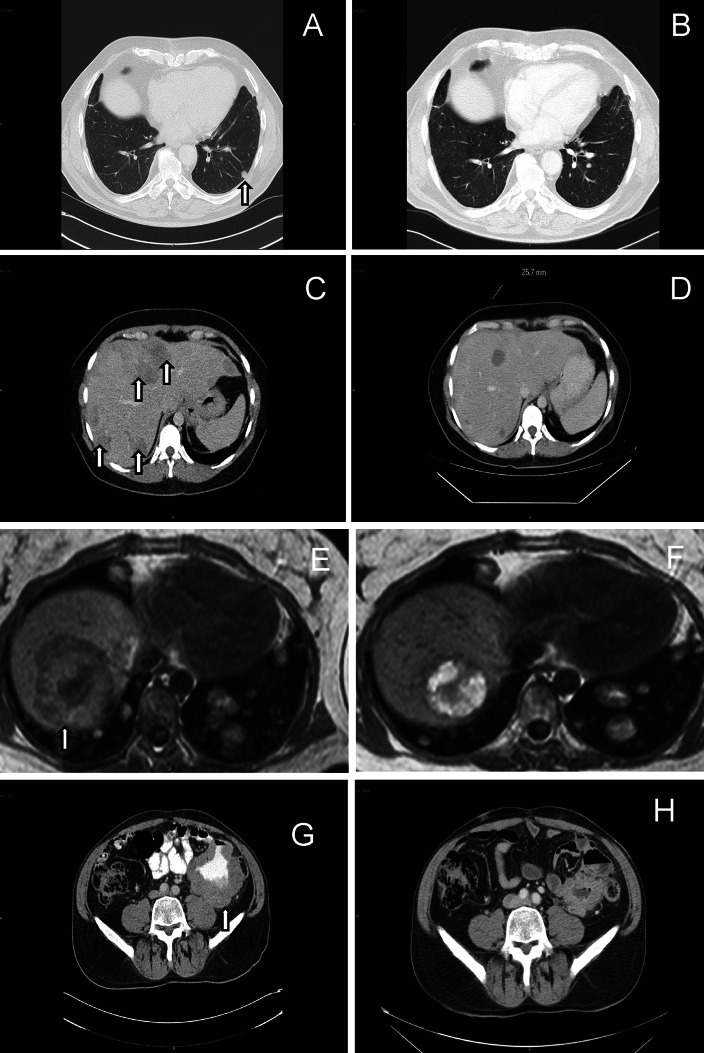
Adoptive transfer of rapidly expanded TILs after lymphodepleting preconditioning resulted in 36% objective responses (1 CR; #17 and 3 PR; #16, #19, #22) in patients treated with core biopsy TILs and 7% OR (1 PR, #4) treated with TILs from surgically removed tissue. Scans (CT/MR) before and 1–2 months after treatment in the four partial responders are shown in Fig. 3a–h. For the patients experiencing a partial response, tumor progression was demonstrated 3 (#19), 3.5 (#4), 4 (#16) and 4.5 (#22) months after treatment. For patient #16, the response was followed by normalization of high pretreatment levels of transaminases, lactate dehydrogenase and the tumor marker S-100. In addition, tumor regression was demonstrated in a further 6 patients without reaching the criteria for partial responses. Most patients experienced minor side effects from IL-2 treatment, most commonly fever. No autoimmune side effects were observed.
Fig. 3.
Partial responses in patients #4 (a–b), #16 (c–d), #19 (e–f) and #22 (g–h) demonstrated with scans (CT/MR) after treatment of metastatic malignant melanoma with lymphodepletion, autologous T cells obtained by ultrasound-guided core biopsy and low-dose s.c. IL-2. Arrows indicate tumor lesions pretreatment
Discussion
Here, we report the first attempt to isolate and treat patients with TILs from core needle biopsies taken from metastatic melanoma lesions. Current protocols developed and refined at the Surgery Branch, NCI, focus solely on surgery. We present an alternative and less invasive method for obtaining tumor tissue that enables TIL growth in clinically relevant numbers. We successfully isolated TILs in 10 of 11 patients who underwent core needle biopsies with approximately 1/3 of the populations showing IFNγ activity. We found no statistical correlations between the number of core biopsies and TIL growth or specificity in this small study. However, 3 core biopsies could be considered as the absolute minimum number of biopsies needed to generate relevant amounts of TILs. This amount allows for both analysis and rapid expansion using anti-CD3 antibody and IL-2 to produce therapeutically relevant numbers of cells for ACT. We assessed the anti-tumor responses by CT scans 1 month after treatment. However, late responses several months after treatment are sometimes seen in cancer patients treated with specific immunotherapy [12]. In one of our patients, a partial response was demonstrated 1 month after treatment at the regular CT examination, but the response was more pronounced according to a CT scan 2 months post T-cell infusion. In another patient, a partial response was obtained at 2 months after treatment but not after 1 month. Herein, most patients were not examined with more than one scan after therapy. If we had scheduled the first post-treatment CT scan some weeks later, and/or if several post-treatment scans had been mandatory, more patients might had been classified as responders.
The majority (4/5) of the patients experiencing an objective response were treated with cells obtained from core biopsy. This indicates that the likelihood of treatment benefit is not greater with T cells prepared from the more complicated procedure of tumor surgery. Surgery allows for larger volumes of tissue which is directly correlated to the numbers of tumor-infiltrating immune cells [13]. However, surgery also entails a great deal of discomfort and exposes patients to the risk of severe complications, e.g., infections and bleeding. Core needle biopsy is considerably less expensive to perform, requires only local anesthesia, takes a few minutes to complete, leaves no scar tissue and allows most patients to rapidly resume normal activity. It also allows sampling of metastases that are inaccessible to surgery. We have in a previous publication reported that tissues obtained by surgery are more likely to contain TILs. We could, however, in that preclinical study not find any differences other than sheer cell numbers as the reactivity of isolated TILs was comparable with both methods [7]. One disadvantage with the limited tissue recovery that core needle biopsies entail is the disability to create autologous tumor cell lines for specificity analysis of TILs. In order to conduct this analysis, one must rely on stimulation of TILs with HLA semi-matched melanoma cell lines and accept the shortcomings of that [7]. The biopsy procedure also means less tissue for histopathological analysis.
Of note is that the administration of low dose s.c. IL-2 in the current trial is clearly less toxic than the i.v. use of this cytokine in high dose [14]. This means shorter hospitalization, and compliance also for elderly patients and patients with lower performance status. Another advantage of using low dose s.c. is the lower cost. The patient in whom a CR was demonstrated is still alive with no signs of tumor relapse 3 and a half year after treatment. He is still on low-dose s.c. IL-2. A link between successful immunotherapy and the onset of autoimmunity has been noted in several clinical immune based therapies. However, no clinical autoimmune side effects were observed in this study. We found no correlation between the number of infused cells and clinical response in this study. In fact, the patient experiencing the most pronounced PR did only receive 3.0 × 109 cells.
We conclude that core needle biopsy of melanoma lesions is a reliable method for generation of relevant numbers of TILs for therapy. This approach is highly pertinent in cases where surgery is not applicable and will facilitate the accessibility and dissemination of current protocols for TIL generation to multiple centers. For the first time, objective responses were demonstrated in malignant melanoma patients after treatment with TILs obtained by needle biopsy. In contrast to previous studies, the treatment was combined with low-dose, instead of high-dose IL-2. The study is, however, too small to assess the true clinical benefit of this concept.
Acknowledgments
We are grateful to all patients. The Research Foundation Stiftelsen Onkologiska Klinikens i Uppsala Forskningsfond has kindly supported this study. This study was supported by grants to Prof. T. H. Tötterman from the Swedish Cancer Society, Uppsala University and Hospital (ALF) and Lion’s Cancer Fund.
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- ACT
Adoptive cell therapy
- CM
Complete media
- CT
Computed tomography
- IFN
Interferon
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- HLA
Human leukocyte antigen
- IL-2
Interleukin 2
- PEST
Penicillin-streptomycin
- TIL
Tumor-Infiltrating lymphocyte
Footnotes
Gustav J. Ullenhag and Arian M. Sadeghi contributed equally to this paper.
References
- 1.Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987;138(3):989–995. [PubMed] [Google Scholar]
- 2.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, Shalmon B, Hardan I, Catane R, Markel G, Apter S, Ben-Nun A, Kuchuk I, Shimoni A, Nagler A, Schachter J. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 6.Lindgren PG. Percutaneous needle biopsy. A new technique. Acta Radiol Diagn (Stockh) 1982;23(6):653–656. doi: 10.1177/028418518202300621. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson B, Sadeghi A, Bengtsson M, Wagenius G, Totterman TH. Effector T cell analysis of melanoma tumor-infiltrating lymphocyte cultures using HLA-ABC semimatched melanoma cell lines. J Immunother. 2008;31(7):633–643. doi: 10.1097/CJI.0b013e3181822097. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson B, Cheng WS, Totterman TH, Essand M. Ex vivo stimulation of cytomegalovirus (CMV)-specific T cells using CMV pp65-modified dendritic cells as stimulators. Br J Haematol. 2003;121(3):428–438. doi: 10.1046/j.1365-2141.2003.04300.x. [DOI] [PubMed] [Google Scholar]
- 9.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128(2):189–201. doi: 10.1016/0022-1759(90)90210-M. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff SL, Smith FO, Klapper JA, Sherry R, Wunderlich JR, Steinberg SM, White D, Rosenberg SA, Dudley ME, Yang JC. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother. 2010;33(8):840–847. doi: 10.1097/CJI.0b013e3181f05b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartzentruber DJ. High-dose interleukin-2 is an intensive treatment regardless of the venue of administration. Cancer J. 2001;7(2):103–104. [PubMed] [Google Scholar]