Fig. 4.
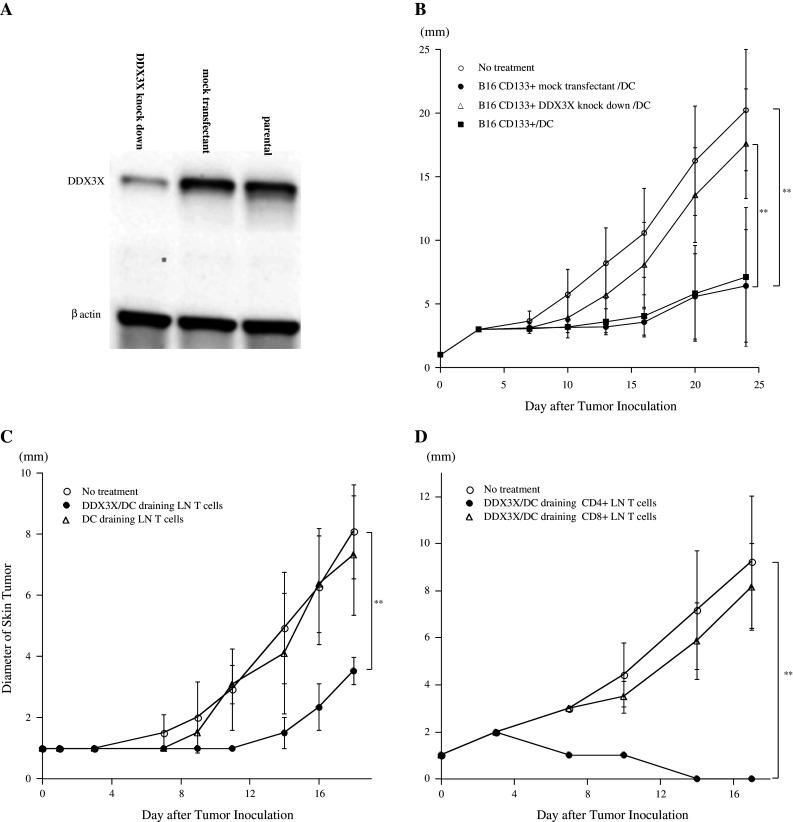
a Immunoblotting analyses of DDX3X expression in DDX3X-knockdown CD133+ B16 cells, mock-shRNA CD133+ B16 cells, and CD133+ B16 cells. b Protective antitumor immunity induced by DDX3X-knockdown CD133+ B16 tumor cell vaccination was significantly inferior to that induced by mock-shRNA CD133+ B16 or CD133+ B16 tumor cell vaccination. A total of 5,000 cGy-irradiated mock-shRNA and DDX3X knockdown CD133+ B16 cells were co-cultured with DCs for 8 h. One million CD11c+ cells purified with CD11c microbeads and autoMACS™ were subcutaneously administered to B6 mice. Two weeks after immunization, mice were subcutaneously inoculated along the midline of the abdomen with 2 × 106 parental B16 cells. Each group contained 5 mice. *P <0.05 and **P <0.01. c Efficacy of the antitumor therapeutic effect mediated by DDX3X-draining LN T cells. DCs were pulsed with synthesized DDX3X protein at 5 μg/mL for 8 h and isolated as CD11c+ cells. CD62Llow T cells were isolated as antigen-primed T cells from LNs draining DDX3X/DC or DCs. LN T cells were cultured for 5 days as described in the “Materials and methods” and intravenously infused into the mice bearing 2-day-established B16 subcutaneous tumors after sublethal whole body irradiation (500 cGy). Ten million T cells were infused intravenously. Each group contained 5 mice. *P < 0.05 and **P < 0.01. d Efficacy of the antitumor therapeutic effect mediated by DDX3X-draining CD4+ or CD8+ LN T cells. CD62Llow LN T cells were isolated as antigen-primed T cells and were further purified as CD4+ or CD8+ T cells with magnetic beads, following the manufacturer’s instructions. After the LN T cells were purified, they were cultured for 5 days, as described in the “Materials and methods”, and intravenously infused into the mice bearing 2-day-established B16 subcutaneous tumors after sublethal whole body irradiation (500 cGy). Ten million T cells were infused intravenously. Each group contained 5 mice. *P < 0.05, and **P < 0.01
