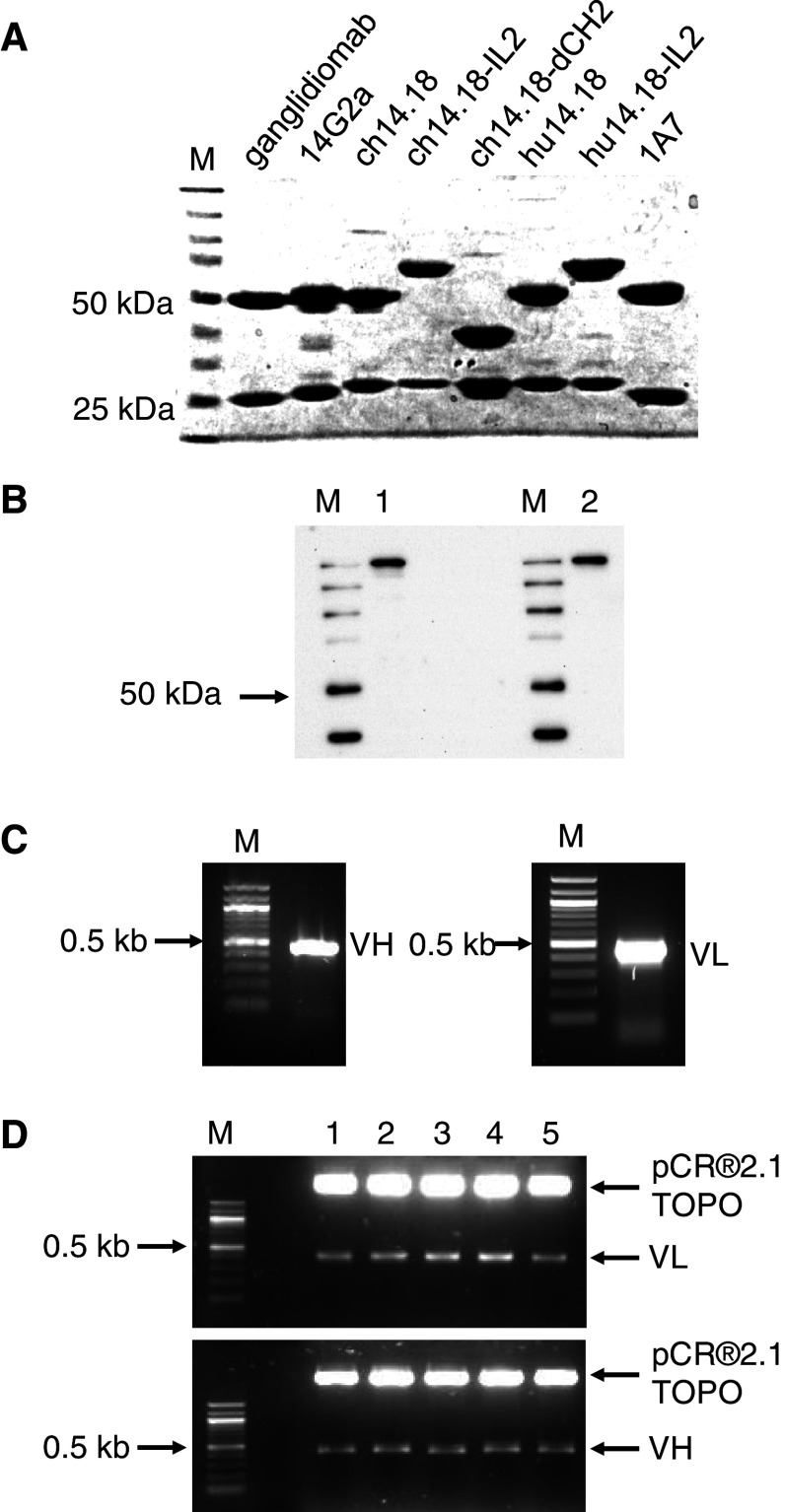
Fig. 3.
Molecular characterization of ganglidiomab. a Integrity of immunoglobulins used in this study was analyzed by 8 % SDS polyacrylamide gel electrophoresis under reducing conditions. b The protein structure of ganglidiomab was analyzed by Western blot following SDS–PAGE under non-reducing conditions (a) from cell lysates of ganglidiomab-producing hybridoma cells (1) as well as in the supernatant (2) using anti-GD2-antibody ch14.18/CHO as detection antibody (M Precision Plus Protein standard). c The coding sequences of variable heavy and light chains of ganglidiomab were amplified by RT-PCR as described in “Materials and methods.” PCR products were analyzed by agarose gel electrophoresis. M: 100-bp ladder. d PCR products were cloned into pCR®2.1-TOPO plasmids and analyzed by an EcoRI restriction enzyme digest. 1–5 individual clones, M 100-bp ladder