Abstract
Previously, we developed a clinically relevant therapy model for advanced intracerebral B16 melanomas in syngeneic mice combining radiation and immunotherapies. Here, 7 days after B16-F10-luc2 melanoma cells were implanted intracerebrally (D7), syngeneic mice with bioluminescent tumors that had formed (1E105 to 7E106 photons per minute (>1E106, large; <1E106, small) were segregated into large-/small-balanced subgroups. Then, mice received either radiation therapy alone (RT) or radiation therapy plus immunotherapy (RT plus IT) (single injection of mAbPC61 to deplete regulatory T cells followed by multiple injections of irradiated granulocyte macrophage colony stimulating factor transfected B16-F10 cells) (RT plus IT). Radiation dose was varied (15, 18.75 or 22.5 Gy, given on D8), while immunotherapy was provided similarly to all mice. The data support the hypothesis that increasing radiation dose improves the outcome of immunotherapy in a subgroup of mice. The tumors that were greatly delayed in beginning their progressive growth were bioluminescent in vivo—some for many months, indicating prolonged tumor “dormancy,” in some cases presaging long-term cures. Mice bearing such tumors had far more likely received radiation plus immunotherapy, rather than RT alone. Radiotherapy is a very important adjunct to immunotherapy; the greater the tumor debulking by RT, the greater should be the benefit to tumor immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-015-1772-7) contains supplementary material, which is available to authorized users.
Keywords: Radiation therapy, Radiation dose, Immunotherapy, Intracerebral melanoma, Tumor dormancy
Introduction
Of ~200,000 brain tumor cases in the USA yearly, most (~160,000) are metastatic—deriving primarily from lung, breast, or melanoma tumors [1, 2]. Despite advances in radiation therapy (RT), median survival is still measured in months. Therefore, there is much interest in combining radiation with additional therapies including chemo- and immunotherapies. In both pre-clinical and clinical studies, the combination of active immunotherapy (vaccination) and RT (RT plus IT) is now shown to be more effective than RT alone in treating advanced central nervous system (CNS) tumors [e.g., 3–5]. The challenge now is to make active tumor immunotherapy (IT) widely available, effective, and durable. In this paper, we show that increasing RT dose greatly improves the outcome of immunotherapy in a mouse therapy model of advanced intracerebral melanoma.
Many mechanisms have been attributed to the emergence of distant metastases from metastatic dormancy, after the removal of the primary tumor; angiogenesis, immune surveillance, tumor environment (e.g., extracellular matrix, inflammatory signals, epigenetics, genetic alterations) [6–12] can all influence the escape of many types of tumors, including breast, prostate, and melanoma, from metastatic dormancy to tumor progression, a process that can take years to decades. Cancer therapies (chemo-, radiation-, and immunotherapies) cause the lengthening of the time between treatment and clinical recurrence—here, therapy-induced dormancy. A large fraction of local breast cancer recurrences after surgery and radiation occur 5–10 years after therapy [13]. Therapeutic interventions that can significantly lengthen or make permanent the dormant state would have beneficial effects on tumor-free progression and long-term survival [14].
Radiation therapy of tumors can induce a progression-free period of stable disease (i.e., dormancy) by inducing lethal double strand breaks in the DNA of cells within the tumor either by directly damaging the DNA or indirectly, by generating reactive oxygen species [15, for a recent review]. Cells in the tumor microenvironment, e.g., tumor, endothelial, stromal, macrophage that have extensive DNA damage usually undergo programmed cell death that further modifies the tumor microenvironment. Chemotactic signals from dying cells, upregulated adhesion molecules, and stress molecules on intact and injured endothelial cells call in immune cells and facilitate dendritic cell maturation. Activated immune cells release cytokines that up-regulate major histocompatibility complex (MHC) expression on tumor cells, which in turn increase the tumor cells’ ability to be recognized by T cells. Dying tumor cells are captured by mature dendritic cells, which then migrate to draining lymph nodes where T cell activation against tumor antigens may occur. The tumor environment becomes an evolving mix of stimulatory and suppressive mechanisms affecting tumor composition, angiogenesis and immune modulation [Nicely described, 16].
Koebel et al. [17] showed that in addition to the immune system’s capacity to kill cancer cells and alter tumors by virtue of immune escape, the immune system can restrain the outgrowth of small stable (“dormant”) tumors in methylcholanthrene (MCA)-treated immunocompetent mice by establishing an “equilibrium” state in which the lack of net expansion of these dormant tumors is best explained by cytostatic and cytolytic immune effects. Liang et al., using a subcutaneous tumor model, reported that radiation therapy induces periods of tumor dormancy characterized by both actively proliferating tumor cells and pronounced immune infiltration causing immune-mediated apoptosis of tumor cells, and resulting in a radiation-induced tumor equilibrium [18]. In this study, we have monitored advanced intracerebral weakly immunogenic, aggressive melanomas by bioluminescence after treatment with the combination of RT plus IT. IT consisted of a single partial depletion of regulatory T cells (Tregs), followed by a series of four weekly injections of irradiated B16-F10 cells previously transfected to express granulocyte macrophage colony stimulating factor (GM-CSF). We showed that such IT induces tumor-directed T cell responses [3]. RT dose was varied, while the IT administered remained the same. As RT dose increased, IT outcome improved in a subset of mice; periods of tumor dormancy increased and long-term cures were elicited. The study underscores the need for methods to increase radiation debulking to improve the outcome of IT. Further, our model should be very useful for the study of therapy-induced brain tumor dormancy.
Materials and methods
Cell culture
B16-F10-luc2 cells obtained from Perkin Elmer (Akrin, Ohio) were grown on Sarstedt tissue culture Flasks (Newton, NC) in DMEM-CM (GIBCO #11995) supplemented with glutamine (2 mM), Penn/Strep (100 U/ml penicillin; 100 μg/ml streptomycin), and Fungizone (0.25 μg/ml), all from Invitrogen (Grand Island, NY).
In vivo experimentation
All of the work and study protocols performed were approved by the University of Connecticut Health Center Animal Care and Use Committee.
Mouse anesthesia
Briefly, C57BL/6 mice (Charles River), each weighing approximately 18 to 20 g, were anesthetized by intraperitoneal injection of approximately 0.06 ml/20 g of a Ketamine/Xylazine mixture containing 1.1 ml phosphate-buffered saline, 0.10 ml Ketamine solution (100 mg/ml, Lederle Parenterals, Inc., Carolina, PR), and 0.11 ml Xylazine solution (20 mg/ml, Ben Venue Labs, Ridgefield, CT).
Mouse euthanasia
The euthanasia procedure, CO2 narcosis, is done using the Euthanex system, a method approved in Guidelines on Euthanasia by the American Veterinary Medical Association and by the University of Connecticut Health Center Animal Care Committee (IACUC).
Intracerebral tumors
Cultured untreated B16-F10-luc2 cells (250) were mixed with 5000 disabled B16 cells that were rendered non-clonogenic by x-irradiation (100 Gy) and the mixture was injected into the brains of isogenic C57BL/6 mice (Charles River, Kingston, NY) in 1.0 μl of culture medium to initiate intracerebral melanoma tumors, as described previously [3]. Untreated, mice had to be euthanized 13–20 days post-implantation (median ~day 15.5), when the mice showed signs of imminent death from a large intracerebral melanoma by failing the flip test (i.e., inability to instantly right itself when flipped).
Irradiations
Irradiations were performed at the Philips RT100 X-ray facility located at Brookhaven National Laboratory (Upton, NY) 8 days after tumor implantation, as described previously [3].
Dosimetry
The BNL Medical Department Phillips RT-100 was operated at 100-peak kilovoltage (kVp), 8 mA and the beam was filtered with 1.7 mm aluminum, resulting in a median beam energy of 37 keV. The source-to-aperture distance was 10 cm. The 3.5-mm-thick shield effectively blocked beam penetration. The dose rate was calculated to be a nominal 7.6 Gy/min, using an absolute calibration of a Radcal ion chamber and its electrometer, and 2-min measurements of the dose in the ion chamber and the electrometer prior to each session.
Immunotherapy
Immunotherapy was performed as described previously [3], except in these experiments mice received a total of four weekly injections of 106 irradiated (100 Gy) granulocyte macrophage colony stimulating factor (GM-CSF)-transfected B16 cells (B16-GM-CSF) (kindly provided by Dr. Glenn Dranoff, Harvard Medical School, Boston, MA.)
Quantification of intracerebral B16-F10 tumors using in vivo luciferase bioluminescence
Approximately 250 B16-F10-luc2 cells were mixed with 5000 irradiated (~100 Gy) B16 cells and implanted in the brains of C57BL/6 mice. After 7 days, the brains of the mice were imaged using an IVIS Spectrum In Vivo Imaging System (Caliper Life Sciences, Hopkinton, MA). Briefly, mice were anesthetized with 2 % isoflurane plus oxygen, shaved, and imaged for 120, 60, 30, 15, 5, and 1 s, 13 min following an sc injection of 75 mg/kg D-Luciferin (Caliper Life Sciences, Hopkinton, MA). Photons per minute (photons/min) were obtained after selecting a region of interest that encompassed the portion of the head containing the tumor.
Implantation of mouse brain tumor regions into the flanks of Rag1 knockout mice and histology
Brains from the four long-term surviving mice that received 22.5 Gy-only, and the two long-term surviving mice that received 18.75 Gy plus IT, that had the greatest luciferase signal after 1 year, were dissected after euthanasia. A coronal slice was made through the original site of tumor implantation that was determined visually. A region of brain in the vicinity of tumor implantation, approximately 2–3 mm in each dimension, was excised from one of the two brain pieces and cut into several smaller pieces. A small incision was made on the flank of Rag1 knockout mice that lack mature B and T cells (Jackson Labs B6.129S7-Rag1tm1Mom/J). A pair of forceps was used to open the flap, the brain pieces were inserted, and the flap was sealed with several microliters of glue. The mice were observed for sc tumor growth for 3 months. The remaining brain piece was fixed in buffered formalin, embedded in paraffin, and sectioned and stained with H&E. Tiled epifluorescent (DAPI) and brightfield (H&E) images were acquired with a Plan-Apochromat 20X/0.8 M27 objective on the Zeiss Axio Scan.Z1 microscope.
Interpretation of data: survival curves
A survival curve for each group was estimated using the Kaplan–Meier method. The log rank test was used to compare two survival curves among the treatment groups. Some animals lived for a year and were right censored at 365 days. Analyses were conducted using the LIFETEST procedure in the Statistical Analysis System (SAS) software suite, except that we have reported a one-sided p value for each pairwise comparison [control (C) vs RT, C vs RT plus IT, and RT vs RT plus IT].
Tumor size
Wilcoxon nonparametric rank sum analysis, appropriate for small groups of animals, was used to assess the likelihood that tumors in the two groups of mice were of the same or different size.
Results
Immunotherapy (IT) combined with 15 Gy radiation therapy (RT) increases the time to tumor progression (TTP) of intracerebral melanoma tumors compared to RT alone
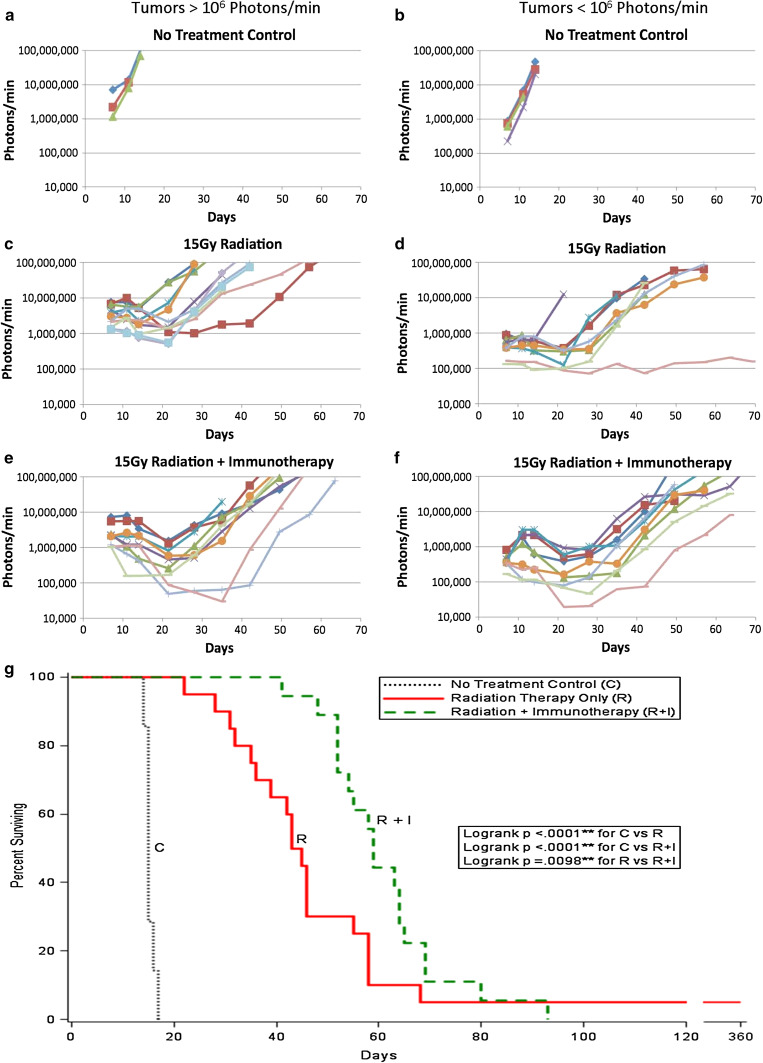
RT was performed 8 days after implantation of 200 B16-F10-luc2 cells in the brains of C57BL/6 mice. A single X-ray dose of 15 Gy was given as previously described [3]. Figure 1 shows the growth of the tumors with time as followed by luciferase luminescence. On day seven, 1 day prior to irradiation, the mice were imaged and the tumors sorted according to size to create experimental groups of roughly equivalent size distributions. Tumors >1,000,000 photons/min (>106 photons/min) are shown in Fig. 1a, c, e; tumors smaller than 1,000,000 photons/min (<106 photons/min) are shown in Fig. 1b, d, f. Figure 1a, b represents tumors growing in mice that received No Treatment (NT). Tumors progressed without delay, and the median day of death was ~15 days [3]. Figure 1c, d represent mice that received RT-only. Tumors generally decreased in size and then, after a delay, resumed progression. Figure 1g and Table 1 show that median survival was extended to day 44 due to a single radiosurgical dose of 15 Gy, but 95 % of mice succumbed to their tumors by day 68. Figure 1e, f shows the fate of tumors in mice that received 15 Gy RT on day eight plus immunotherapy (IT) as previously described [3]; briefly, Tregs were partially depleted by a single ip injection of 0.5 mg mAbPC61 followed by a series of four weekly subcutaneous injections of irradiated (100 Gy) GM-CSF-secreting B16-F10 cells given on days 9, 16, 23, 30 following tumor cell implantation; irradiated (100 Gy) GM-CSF transfected B16-F10 cells, when grown in tissue culture, continued to secrete equivalent amounts of GM-CSF as their unirradiated counterparts for 4 days after irradiation (unpublished results). Figure 1g and Table 1 show that median survival was significantly increased to day 59 by the addition of IT, but again all mice eventually died of their tumors after 3 months, as was found previously. Moreover, the percent surviving increased with the addition of IT (p < 0.0098). Median life extension due to IT was increased for both smaller tumors (46D to 64D) (Suppl. Fig. S1B) and larger tumors (43D to 55D) (Suppl. Fig. S1A), although statistical significance (increased percent surviving rate) was only reached for the larger tumors (p = 0.0043). Interestingly, when comparing the effects of RT-only on large and small tumors (Suppl. Fig. S1C), there was no statistical significance; about 40 % of the mice with small tumors showed additional life extension. RT plus IT was more effective when smaller tumors were treated (p = 0.034) (Suppl. Fig. S1D), as the median was shifted from 56D (>106 photons/min) to 64D (<106 photons/min). Hence, the combination of RT plus IT was more effective when treating smaller tumors than larger tumors. Suppl. Fig. S2 and Table 1 showed that for all tumors, TTP for RT plus IT-treated mice increased significantly when compared to 15 Gy RT-only (p < 0.013) (median times shifted to 20D from 13.5D). Comparisons of RT-only (15 Gy) versus RT plus IT for small and large tumors (Suppl. Fig, S3A, B), both showed increases in TTP but statistical significance was only obtained for large tumors (p = 0.013). Comparisons of small and large tumors treated by RT-only (Suppl. Fig. S3C) or RT plus IT (Suppl. Fig. S3D) also showed differences (p < 0.033; p < 0.066, respectively). Hence the combination of RT plus IT increased the TTP compared to tumors in mice that received RT-only.
Fig. 1.
Advanced intracerebral (ic) B16-F10-luc2 melanomas are followed by luciferase bioluminescence as a function of time following the implantation of 250 B16-F10-luc2 cells and either no treatment, RT-only or RT plus IT. a, b, No treatment controls (N = 7); c, d, 15 Gy RT-Only (N = 20), e, f, 15 Gy RT plus IT (N = 18). a, c, e Tumors >106 photons/min measured 7 days after ic tumor cell implantation. b, d, f Tumors <106 photons/min measured 7 days after ic tumor cell implantation. Each line represents a different mouse. The data represent two separate experiments that have been combined. g The combination of RT plus IT significantly extends median survival over that provided by RT alone. Shown are the survival plots for all tumors (both >106 and <106 photons/min measured on day 7 following ic implantation. (Black dots no treatment control, solid red 15 Gy RT-only, dashed green 15 Gy RT plus IT)
Table 1.
Summary of median time to tumor progression (TTP) (days), median survival (days), and fraction long-term survival for untreated controls, 15 Gy RT only and 15 Gy RT Plus IT
| Untreated control (N = 7) |
15 Gy R ALL (N = 20) |
15 Gy R + I ALL (N = 18) |
p | 15 Gy R >106 P/min (N = 13) |
15 Gy R + I >106 P/min (N = 11) |
p | 15 Gy R <106 P/min (N = 7) |
15 Gy R + I <106 P/min (N = 7) |
p | 15 Gy RL >106 P/min (N = 13) |
15 Gy RS <106 P/min (N = 7) |
p | 15 Gy R + IL >106 P/min (N = 11) |
15 Gy R + IS <106 P/min (N = 7) |
p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Time to tumor progression (TTP) (days) | N/A |
13.5 (7–28) |
20 (13–34) |
0.013 |
14 (6–26) |
19 (14–32) |
0.013 |
18 (6–00) |
20 (19–27) |
0.142 | 13 | 18 | 0.033 | 18 | 20.5 | 0.066 |
| Median survival (days) | 15 |
44 (22–360) |
59 (41–93) |
0.0098 |
43 (28–58) |
55 (41–80) |
0.0043 |
46 (22–360) |
64 (52–93) |
0.195 | 1 + 3 | 46 | 0.14 | 56 | 64 | 0.034 |
| Fraction long-term survivors | 0 | 1/20 | 0/18 | 0/13 | 0/11 | 1/7 | 0/7 | 0/13 | 1/7 | 0/11 | 0/7 |
All = All tumors (large [L] + small [S]); large tumors >106 photons [P]/min; small tumors <106 photons [P]/min; P = p value
Increasing the radiation dose from 15 to 22.5 Gy increases tumor dormancy and the efficacy of immunotherapy
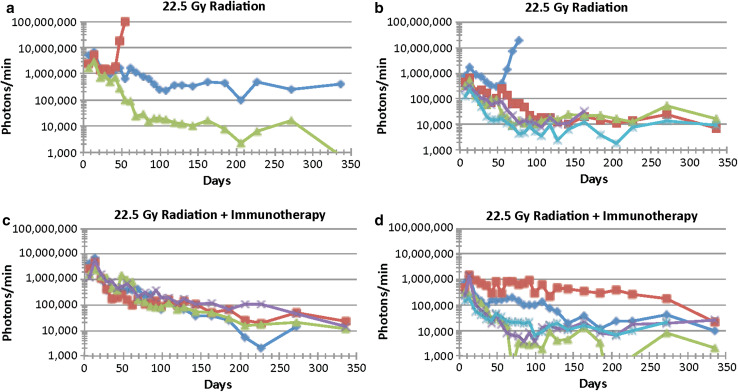
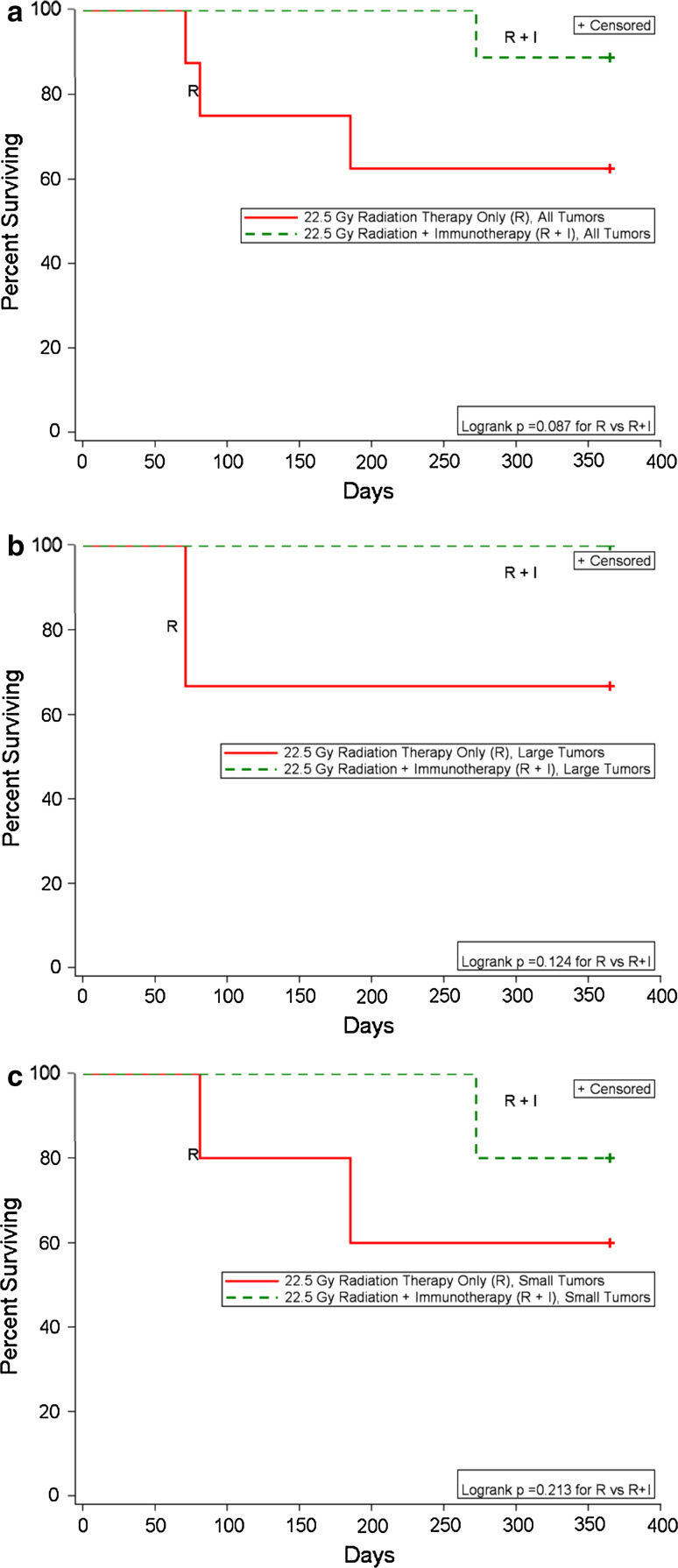
The experiment described above was repeated except the RT dose was increased from 15 to 22.5 Gy. The results are shown in Figs. 2, 3. Again, larger tumors are shown in Fig. 2a, c, while smaller tumors are shown in Fig. 2b, d. One of the three larger tumors that received RT-only (22.5 Gy) progressed (Figs. 2a, 3b); TTP and death were D40, D71, respectively. Of the five smaller tumors that received RT-only, two progressed (Figs. 2b, 3); TTP and death were D47, D81 and D140, D185, respectively. (Note: The mouse depicted by purple x was not imaged, as it was found dead with a tumor in its brain on the day of imaging). Of the four mice with larger tumors that were treated with RT plus IT, one was euthanized on day 290 due to neurological signs, but no tumor was observed by bioluminescence or histological analysis. Since death was not due to a recurrent tumor, death of this mouse was not shown in Fig. 3. Of the five mice with smaller tumors that were treated with RT plus IT, one developed a tumor (TTP not known) and was euthanized on day 272. The data suggest that when a higher radiation dose was used (22.5 Gy), IT combined with RT prevented four of four of the larger tumors from progressing, while one of the three tumors progressed in the RT-only group. Two out of five of the smaller tumors progressed in the RT-only group, and one out of five in the RT plus IT group progressed at a significantly later time (Fig. 3a–c). Hence, IT appears to prolong the time the tumors remain dormant prior to progressing, although given the low numbers of tumors that progress, many more mice would be needed in each group to establish statistical significance.
Fig. 2.
Advanced intracerebral (ic) B16-F10-luc2 melanomas are followed by luciferase bioluminescence as a function of time following the implantation of 250 B16-F10-luc2 cells and either 22.5 Gy RT alone or 22.5 Gy RT plus IT. a, b 22.5 Gy RT-Only (N = 8), c, d 22.5 Gy RT plus IT (N = 9). a, c Tumors >106 photons/min measured 7 days after ic tumor cell implantation. b, d Tumors <106 photons/min measured 7 days after ic tumor cell implantation. Each line represents one mouse. The data represent the results of a single experiment. Note: For the mouse represented by the purple X, the last bioluminescent assay was day 162 at which time the signal was starting to rise. Death occurred on D180 and TTP was ~D140. For the mouse represented by the light blue X, the last bioluminescent assay was done on D225. Death occurred on D272 and TTP was ~D225
Fig. 3.
Survival plots for the data shown in Fig. 2: RT plus IT increases the number of long-term survivors compared to RT alone. a Shown are the survival plots for all tumors (both >106 and <106 photons/min measured on day 7 following ic implantation). Solid red 22.5 Gy RT-only, dashed green 22.5 Gy RT plus IT. b Survival plots for tumors >106 photons/min measured on day 7 following ic implantation. Solid red 22.5 Gy RT-only, dashed green 22.5 Gy RT plus IT. c Survival plots for tumors <106 photons/min measured on day 7 following ic implantation. Solid red 22.5 Gy RT-only, dashed green 22.5 Gy RT plus IT
The intermediate radiation dose of 18.75 Gy also increases tumor dormancy prolongation by immunotherapy
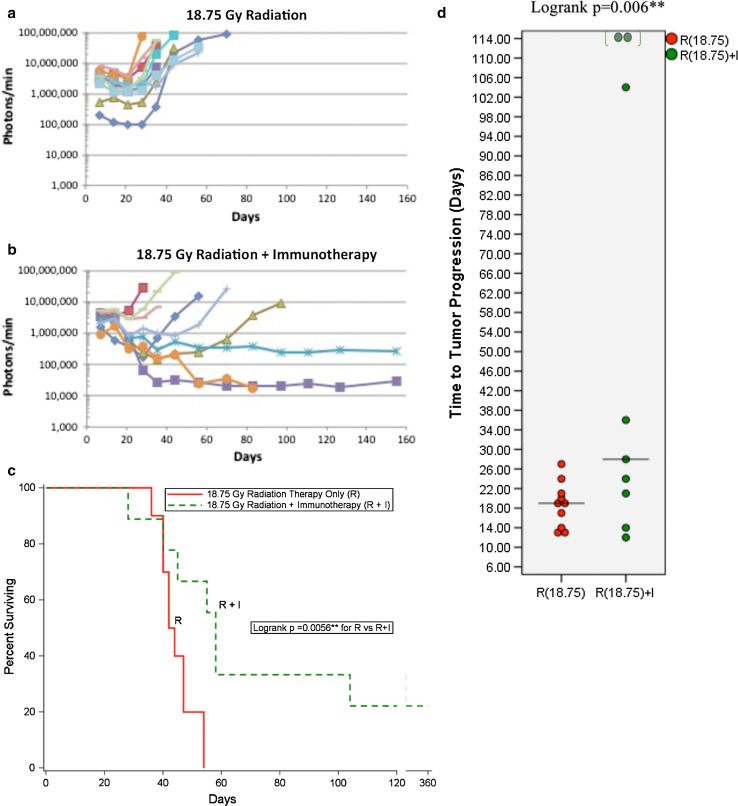
Tumor growth after an intermediate RT dose of 18.75 Gy is shown in Fig. 4a. All of the tumors ultimately progressed and compromised their hosts. Tumor growth after 18.75 Gy RT plus IT is shown in Fig. 4b. Two of the nine tumors failed to progress. The survival curves are shown in Fig. 4c, Table 2. The survival curve for RT plus IT was clearly better than RT-only (p = 0.0056). Median survival was increased ~2 weeks, from D43 (RT-only) to D58 (RT plus IT). The distribution of TTP shifted higher from the 18.75 Gy RT-only to RT plus IT (p = 0.006) where the median of TTP also increased from 19D (RT-only) to 28D (RT plus IT) (Fig. 4d). A direct comparison of 15 and 18.75 Gy for RT-only (Suppl. Fig. S4A, C) and RT plus IT (Suppl. Fig. S4B, D) supports the contention that the increased radiation dose when combined with immunotherapy greatly benefits a third of the mice. An intermediate radiation dose, such as 18.75 Gy, would be an ideal RT dose to use to study therapy-induced tumor dormancy in this system, both in the absence and presence of IT.
Fig. 4.
Advanced intracerebral (ic) B16-F10-luc2 melanomas followed by luciferase bioluminescence as a function of time following the implantation of 250 B16-F10-luc2 cells. a 18.75 Gy RT-only (N = 10) or b 18.75 Gy RT plus IT (N = 9) (all tumors). Each line represents one mouse. The data represent the results of a single experiment. c Survival plots for the data shown in a, b RT plus IT increases both median survival and long-term survivors compared to RT-only. Shown are the survival plots for all tumors (both >106 and <106 photons/min measured on day 7 following ic implantation. (Solid red 18.75 Gy RT-only, dashed green 18.75 Gy RT + IT. d Comparison of the dot plots of TTP for RT-only, RT plus IT for all tumors: RT plus IT increases TTP compared to RT alone. (Red 18.75 Gy RT-only, Green 18.75 Gy RT plus IT
Table 2.
Summary of median time to tumor progression (TTP) (days), median survival (days) and fraction long-term survival for 15 Gy RT only, 15 Gy RT plus IT, 18.75 Gy RT only, 18.75 Gy RT plus IT, 22.5 Gy RT only, and 22.5 Gy RT plus IT
| Untreated control (N = 7) |
15 Gy R ALL (N = 20) |
15 Gy R + I ALL (N = 18) |
p | 18.75 Gy R ALL (N = 10) |
18.75 Gy R + I ALL (N = 9) |
p | 22.5 Gy R ALL (N = 8) |
22.5 Gy R + I ALL (N = 9) |
|
|---|---|---|---|---|---|---|---|---|---|
| Median time to tumor progression (TTP) (days) | N/A |
13.5 (7–28) |
20 (13–34) |
0.013 | 19 | 28 | 0.006 | 40D, 47D, 140D | ~230D |
| Median survival (days) | 15 |
44 (22–360) |
59 (41–93) |
0.0098 | 43 | 58 | 0.0056 | Median not reached | Median not reached |
| Fraction long-term survivors | 0 | 1/20 | 0/18 | 0/10 | 2/9 | 5/8 | 8/9 |
For 22.5 Gy, median survival was not reached. TTP for the three mice that died of brain tumors are listed
Some of the brains of long-term surviving mice have dormant tumors
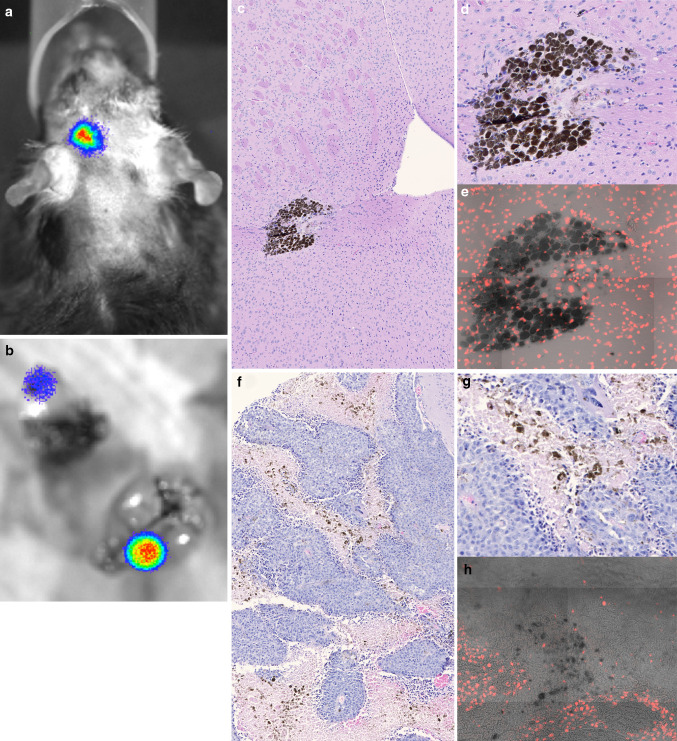
Twelve months after tumor implantation, the brains of four surviving mice with the greatest luciferase signal were dissected and sliced coronally through the site of tumor imaging. Half the brains were processed for histology. The tumor region was dissected from the other half and implanted into the flank of immunologically deficient Rag1 knock-out mice. The two surviving mice that received 18.75 Gy were similarly treated at 10 months. None of the implanted tumors grew, even though the mice had clear evidence of bioluminescence in their brains at the time of killing (Fig. 5a, b).
Fig. 5.
Long-term surviving mouse that received 18.75 Gy RT plus IT contains residual melanin-containing cells in the brain. a Residual bioluminescence at 10 months, prior to euthanasia. b Source of bioluminescent signal is primarily in the brain. c, d, e Nests of residual melanin-producing cells stained with H&E at ×10 (c) and ×20 (d), and DAPI stained at ×20 (e). f, g, h Mouse brain with untreated, control melanoma stained with H&E at ×10 (f), ×20 (g), and DAPI stained at ×20 (h)
Histologically, clusters of melanin-producing cells with a melanoma appearance were found in one of the two long-term surviving mice that had received 18.75 Gy (Fig. 5c) and in two of the four long-term surviving mice that had received 22.5 Gy (Data not shown). These large melanin-producing cells had asymmetric DAPI stained nuclei and an intact, viable appearance (Fig. 5d, e), comparable to sections of brains with control, untreated B16-F10-luc2 tumors (Fig. 5f–h).
Discussion
We have developed a therapy model of advanced intracerebral melanoma in which the combination of 15 Gy RT plus IT was shown to significantly increase median survival over that obtained by RT-only due to a tumor specific, T-cell-dependent immune response [3]. We now show that the same IT is more effective in a subset of mice when given in the context of a higher RT dose. The results, described in Figs. 1, 2, 3, 4, 5 and Suppl. Fig. S1-S4, are summarized in Tables 1 and 2. A number of generalizations can be made: (1) As RT dose was increased from 15 to 18.5 to 22.5 Gy, median survival went from 44D to 43D to ≫135D with their 95 % confidence intervals at (35, 55), (36, 47), and undefined, respectively. Significantly, long-term survivors appeared at 22.5 Gy (66 % of mice with large tumors). Median time to tumor progression (TTP), defined as the time between RT (D8) and the time the tumors began to continuously progress, also increased with increasing RT dose. Median TTP went from 13.5D to 19D to >45D after 15, 18.5 and 22.5 Gy RT; since only a third of the large tumors progressed at 22.5 Gy, median TTP was never reached. (2) Immunotherapy initiated after RT increased both median survival and TTP. At 15 Gy, median survival increased 15D due to the addition of IT, while TTP increased 6.5D, suggesting that one effect of IT is to slow down the rate of tumor growth, possibly by shifting the equilibrium between tumor proliferation and immune-mediated tumor cell death [17, 18]. A similar but less pronounced effect was seen at 18.75 Gy; median survival increased 15D while median TTP increased 9D. (3) At the higher RT, IT resulted in more long-term survivors. Whereas there were only 0–5 % long-term survivors in the RT-only groups, there were 2/9 long-term survivors in the RT plus IT group at 18.75 Gy. At 22.5 Gy long-term survivors (mice that did not die of brain tumors) went from 62.5 %, RT-only to ~80 %, RT plus IT (all tumors). When considering the therapy of the larger tumors, long-term survivors went from 66 to 100 %. (4) In general, just as IT appeared more efficacious when combined with higher doses of RT, it may also be more effective when used to treat the smaller tumors (<106 photons/min) at a single RT dose compared to the larger tumors (≥106 photons/min). While there was little difference in the median survival of mice with either large or small tumors that received RT-only, there was a significant difference in the median survival of mice with either large or small tumors that received RT plus IT. These results suggest that for long-term survival, while some mice with large tumors did better than some mice with small tumors, on average, better results were obtained when treating mice with smaller tumors than mice with larger tumors, both with respect to RT-only and RT plus IT. While these studies used intracerebral melanoma as a model for melanoma metastases to the brain, we hypothesize that these results will be found to apply to primary gliomas as well. Our previous studies with the 9L gliosarcoma led us to a similar hypothesis [19]. Additional studies using a rigorous syngeneic glioma model are needed to test this hypothesis.
There have been many excellent recent reviews on the interplay between RT and the immune system for tumor therapy and the mechanisms involved [e.g., 16, 20–26]. There is mounting evidence that RT can elicit a systemic immune response against tumor cells using a variety of mechanisms, including enhancing the diversity of the T cell receptor repertoire of intratumoral T cells [27]. There is also evidence that RT can enhance the efficacy of vaccine-elicited immune responses to tumors that may be mediated by modifications of the immunosuppressive tumor environment such as myeloid derived suppressor cells and T cell access [28], T regulatory cells [29], among other mechanisms [30]. Unfortunately there are not yet enough data available to define the optimal regimens of RT and IT for different tumor types and locations; questions regarding efficacy of single treatment versus fractionation, radiation dose levels, and the different modes of immunotherapy, especially the use of checkpoint inhibitors, are currently being studied both pre-clinically and clinically [31–35]. In our intracerebral melanoma study, the mechanism(s) by which increased RT dose improved the outcome of IT is not known. Either increased RT dose to the brain tumor had an effect on the immune response per se, or the increased dose helped induce a brain tumor environment whereby the immune response became more effective, or the greater tumor debulking afforded by increased dose was helpful, or a combination of these. Further work will be necessary to define the mechanism(s) underlying the effect in this report. Given the dramatic effects that 18.75 and 22.5 Gy RT had on the mice with advanced intracerebral melanoma when combined with IT compared to 15 Gy, we suggest it is important to develop strategies to improve RT of brain tumors for use with combination therapies such as IT. There have been a number of innovative studies in recent years describing some very powerful RT enhancing strategies including gold nanoparticle-enhanced RT [36] that we hypothesize will increase the efficacy of IT when implemented.
The data we have obtained using 18.5 and 22.5 Gy irradiations suggest this therapy model could also be used to study therapy-induced tumor dormancy. At 22.5 Gy, TTP of tumors that ultimately progressed was between ~40 days and >200 days. After 18.5 Gy RT, most of the tumors progressed after 2–3 weeks; but after adding IT, 25 % of the tumors progressed after ~3 weeks, and one tumor each progressed after 4, 5, and 15 weeks. The long periods in which the tumor cells continue to exhibit bioluminescence but do not progress fit the definition of dormancy, and could be used to dissect out the mechanisms by which the tumors enter and exit dormancy, particularly in the brain environment—whether it is due to balanced proliferation and death or a combination of several mechanisms. This information could potentially be used to devise methods to maintain dormancy for more protracted periods. We have shown that CD4 T cell depletion decreased median survival in RT plus IT-treated mice [3]. We hypothesize that CD4 and/or CD8 T cell depletion will reduce TTP of RT plus IT-treated mice—and possibly some of the RT-only-treated mice as well. It would be interesting to compare TTP after RT in immunocompetent versus immunocompromised mice. Further, it will be possible to ask if other mechanisms contribute to dormancy: throughout dormancy, are new tumor cells being added to replace those killed by the immune system, or do some of the cells enter a quiescent state involving autophagy, senescence, or a combination of the two?
Conclusion
Increasing radiation dose from 15 to 18.75 or 22.5 Gy increased the effectiveness of immunotherapy in a subset of mice, some of which survived long-term with histologically identified dormant tumors in their brains. The therapeutic use of radiation enhancers, therefore, should increase the effectiveness of immunotherapy and prolong tumor dormancy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a University of Connecticut Health Center seed grant to Henry M. Smilowitz.
Abbreviations
- Anti-PD-1
Anti-programmed cell death protein 1 antibody
- C
Control
- CNS
Central nervous system
- D
Day
- DAPI
4′,6-Diamidino-2-phenylindole
- GM-CSF
Granulocyte macrophage colony stimulating factor
- IL
Interleukin
- ip
Intraperitoneal
- IT
Immunotherapy
- kVp
Peak kilovoltage
- mAb
Monoclonal antibody
- MCA
Methylcholanthrene
- MHC
Major histocompatibility complex
- NOD/SCID
Non-obese diabetic/severe combined immunodeficiency
- NT
No treatment
- RT
Radiation therapy
- sc
Subcutaneous
- Tregs
Regulatory T cells
- TTP
Time to tumor progression
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in any form with respect to this article.
References
- 1.Walbert T, Gilbert MR. The role of chemotherapy in the treatment of patients with brain metastases from solid tumors. Int J Clin Oncol. 2009;14:299–306. doi: 10.1007/s10147-009-0916-1. [DOI] [PubMed] [Google Scholar]
- 2.Langley RR, Fidler IJ. The biology of brain metastasis. Clin Chem. 2013;59:180–189. doi: 10.1373/clinchem.2012.193342. [DOI] [PubMed] [Google Scholar]
- 3.Smilowitz HM, Sasso D, Lee E, Goh G, Micca PL, Dilmanian FA. Therapy model for advanced intracerebral B16 mouse melanoma using radiation therapy combined with immunotherapy. Cancer Immunol Immunother. 2013;62:1187–1197. doi: 10.1007/s00262-013-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang HQ, Nuno MA, Richardson JE, Fan X, Ji J, Chu RM, Bender JG, Hawkins ES, Patil CG, Black KL, Yu JS. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossowski L, Aguirre-Ghiso JA. Dormancy of metastatic melanoma. Pigment Cell Melanoma Res. 2009;23:41–56. doi: 10.1111/j.1755-148X.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139–146. doi: 10.1016/j.canlet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–1188. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragado P, Sosa MS, Keely P, Condeelis J, Aguirre-Ghiso JA. Microenvironments dictating tumor dormancy. Recent Results Cancer Res. 2012;195:25–39. doi: 10.1007/978-3-642-28160-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocken M. Early tumor dissemination, but late metastasis: insights into tumor dormancy. J Clin Invest. 2010;120:1800–1803. doi: 10.1172/JCI43424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells A, Griffith L, Wells JZ, Taylor DP. The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res. 2013;73:3811–3816. doi: 10.1158/0008-5472.CAN-13-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, Gray R, Pierce L, Whelan T, Wang Y, Peto R. Effect of radiotherapy after breast conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensel JA, Flaig TW, Theodorescu D. Clinical opportunities and challenges in targeting tumour dormancy. Nat Rev Clin Oncol. 2013;10:41–51. doi: 10.1038/nrclinonc.2012.207. [DOI] [PubMed] [Google Scholar]
- 15.Mladenov E, Magin S, Soni A, Lliakis G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front Oncol. 2013;3:113. doi: 10.3389/fonc.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnette B, Weichselbaum RR. Radiation as an immune modulator. Semin Radiat Oncol. 2013;23:273–280. doi: 10.1016/j.semradonc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 18.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt ME, Weichselbaum RR, Fu Y-X. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–5881. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smilowitz HM, Slatkin DN, Lubimova N, Blattman H, Brauer-Krisch E, Bravin A, Di Michiel M, Stepanek J, Le Duc G, Gebbers J-O, Laissue JA. Synergy of gene-mediated immunoprophylaxis and microbeam radiation therapy for advanced intracerebral rat 9L gliosarcomas. J Neurooncol. 2006;78:135–143. doi: 10.1007/s11060-005-9094-9. [DOI] [PubMed] [Google Scholar]
- 20.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Fomenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein SE, Timmerman R, McBride WH, Schaue D, Hoffe SE, Mantz CA, Wilson GD. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011;2011:439752. doi: 10.1155/2011/439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow KKH, Hara W, Lim M, Li G. Combining immunotherapy with radiation for the treatment of glioblastoma. J Neurooncol. 2015;123:459–464. doi: 10.1007/s11060-015-1762-9. [DOI] [PubMed] [Google Scholar]
- 23.Soukup K, Wang X. Radiation meets immunotherapy—a perfect match in the era of combination therapy? Int J Rad Biol. 2015;91:299–305. doi: 10.3109/09553002.2014.995383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahabi V, Postow MA, Tuck D, Wolchok JD. Immune-priming of the tumor microenvironment by radiotherapy. Am J Clin Oncol. 2015;38:90–97. doi: 10.1097/COC.0b013e3182868ec8. [DOI] [PubMed] [Google Scholar]
- 25.Wattenberg MM, Fahim A, Ahmed MM, Hodge JW. Unlocking the combination: potentiation of radiation-induced antitumor responses with immunotherapy. Radiat Res. 2014;182:126–138. doi: 10.1667/RR13374.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology. 2013;2:e25962. doi: 10.4161/onci.25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filatenkov A, Baker J, Mueller AMS, Kenkel J, Ahn G-O, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S, Shizuru JA, Negrin RN, Engelman EG, Strober S. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res. 2015;21:3727–3739. doi: 10.1158/1078-0432.CCR-14-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persa E, Balogh A, Sáfrány G, Lumniczky K. The effect of ionizing radiation on regulatory T cells in health and disease. Cancer Lett. 2015;368:252–261. doi: 10.1016/j.canlet.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Sridharan V, Schoenfeld JD. Immune effects of targeted radiation therapy for cancer. Discov Med. 2015;19:219–228. [PubMed] [Google Scholar]
- 31.Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: optimal dose and fractionation. Cancer Lett. 2015;368:185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Zeng J, Harris TJ, Lim M, Drake CG, Tran PT. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int. 2013;2013:658126. doi: 10.1155/2013/658126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnette B, Weichselbaum RR. The immunobiology of ablative radiation. Semin Radiat Oncol. 2015;25:40–45. doi: 10.1016/j.semradonc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Mondini M, Nizard M, Tran T, Mauge L, Loi M, Clemenson C, Duque D, Maroun P, Louvet E, Adam J, Badoual C, Helley D, Dransart E, Johannes L, Vozenin MC, Perfettiini JL, Tartour E, Deutsch E. Synergy of radiotherapy and a cancer vaccine for the treatment of HPV-associated head and neck cancer. Mol Cancer Ther. 2015;14:1336–1345. doi: 10.1158/1535-7163.MCT-14-1015. [DOI] [PubMed] [Google Scholar]
- 36.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–N315. doi: 10.1088/0031-9155/49/18/N03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.