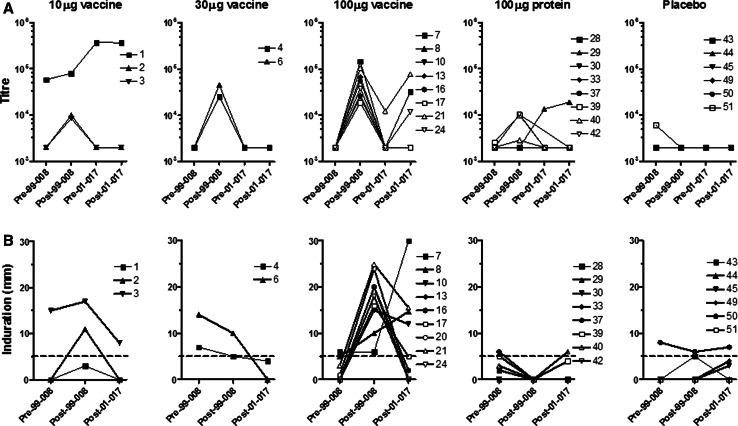
Fig. 1.
Antibody titre and skin DTH in NY-ESO-1 vaccine cohorts. a Antibody titres of individual participants grouped according to dose cohort in the initial vaccination trial (LUD99-008 or 99-008). Y-axis shows reciprocal antibody titre with 2,000 set as the baseline for this assay. Patients received three vaccinations or placebo on the LUD99-008 trial. Pre- and post-vaccination titres are indicated by Pre-99-008 and Post-99-008; Pre-01-017 indicates baseline antibody titre on LUD01-017 trial; Post-01-017 titre assessed 2 or 6 weeks following 1 μg ID NY-ESO-1 protein challenge. b Skin DTH induration measured two days after ID injection of 1 μg of NY-ESO-1 protein. DTH responses for individual participants are grouped according to dose cohort in the initial LUD99-008 vaccination trial. Pre- and post-vaccination DTH size are indicated by Pre-99-008 and Post-99-008; Post-01-017 indicates DTH reaction two days after 1 μg NY-ESO-1 protein challenge on LUD01-017 trial. DTH values below the dotted line (<5 mm) were classified as negative responses