Fig. 1.
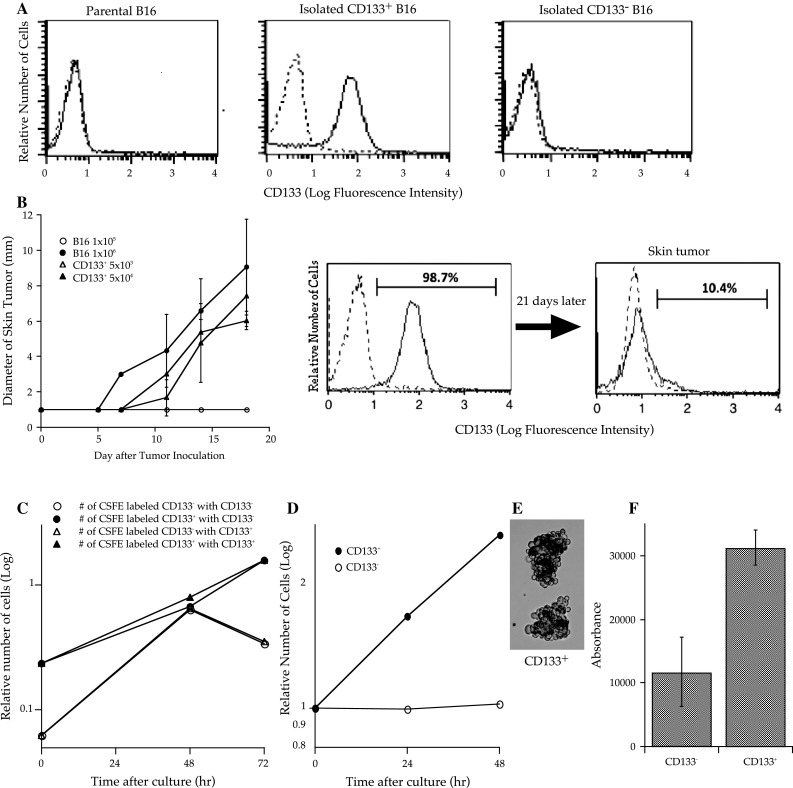
CD133+ B16 melanoma cells demonstrated high tumorigenicity in vivo (each group contained 5 mice) and proliferated in an anchorage- and cell–cell contact inhibition-independent manner in vitro. a One million B16 melanoma cells were stained with phycoerythrin (PE)-conjugated anti-CD133 or PE-conjugated isotype control monoclonal antibodies (mAbs). Dotted lines indicate the fluorescence intensity of tumor cells stained with PE-conjugated subclass-matched isotype control mAbs. Each frame consists of 10,000 cells. b B6 mice were subcutaneously inoculated along the midline of the abdomen with 5 × 103 or 5 × 104 CD133+ cells, or 1 × 105 or 1 × 106 parental B16 cells. The diameter of the skin tumors was measured twice weekly with calipers, and the size was recorded as the average of 2 perpendicular diameters. Each group contained 6 mice. Bars indicate standard deviation. c CD133+ or CD133− tumor cells (0.3 × 106) labeled with carboxyfluorescein diacetate succinimidyl diester (CFSE) were mixed with non-labeled tumor cells (3 × 106) and cultured in complete medium (CM) at 1 × 105 cells/ml. Tumor cells were counted every day and analyzed using a microfluorometer to determine the number of CFSE-labeled tumor cells. Three wells were analyzed for each condition. d, e One million CD133+ or CD133− tumor cells were cultured in 10 ml of CM in 50 ml conical tubes that were rotated to avoid cell attachment. Cell counts were performed every 24 h. After 72 h in culture, CD133+ cells proliferated to build spheroid-like cell aggregates. f The soft agar colony assay was performed using CytoSelect™ 96 Well Transformation Assay (Cell Biolabs Inc.) according to the manufacturer’s instructions; 5 × 103 CD133+ or CD133− B16 tumor cells were cultured in soft agar for 7 days, and colony formation was examined using a 96-well fluorometer
