Abstract
Humoral immune responses against tumor antigens are studied as indirect markers of antigen exposure and in cancer vaccine studies. An increasing number of tumor antigens potentially translated from mutant genes is identified by advances in genomic sequencing. They represent an interesting source for yet unknown immunogenic epitopes. We here describe a multiplex method using the Luminex technology allowing for the detection of antibodies against multiple in silico-predicted linear neo-antigens in large sets of sera. The approach included 32 synthetic biotinylated peptides comprising a predicted set of frameshift mutation-induced neo-antigens. The antigens were fused to a FLAG epitope to ensure monitoring antigen binding to avidin-linked microspheres in the absence of monoclonal antibodies. Analytical specificity of measured serum antibody reactivity was proven by the detection of immune responses in immunized rabbits and a colorectal cancer patient vaccinated with peptides included in the assay. The measured antibody responses were comparable to peptide ELISA, and inter-assay reproducibility of the multiplex approach was excellent (R 2 > 0.98) for 20 sera tested against all antigens. Our methodic approach represents a valuable platform to monitor antibody responses against predicted antigens. It may be used in individualized cancer vaccine studies, thereby extending the relevance beyond the model system in the presented approach.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-014-1595-y) contains supplementary material, which is available to authorized users.
Keywords: Serology, Antibody, Peptide, Multiplex, xMAP technology, Luminex
Introduction
The detection of humoral immune responses has broad diagnostic and scientific implications in the field of infectious diseases, organ transplant immunology, autoimmune diseases, and cancer biology. By the detection of serum antibodies against antigenic epitopes, indirect evidence is gained on the individual’s past and/or current exposure to the antigen. Serology, therefore, is used in diagnosing and monitoring infections such as hepatitis B [1], evaluating donor-directed immune responses in organ transplant recipients [2], and characterizing antibodies directed against self-antigens in order to support the diagnosis of autoimmune syndromes such as rheumatoid arthritis [3].
In cancer biology, the discovery of tumor antigens has drawn attention to humoral immune responses [4, 5]. Tumor antigens are defined as antigenic structures exclusively or predominantly expressed by neoplastic cells. They comprise mutation-derived antigens (e.g., p53) [6], antigens normally exclusively expressed in testis (cancer testis antigens such as NY-ESO-1) [7], antigens with an altered molecular structure in cancer cells (such as MUC1, which is differentially glycosylated) [8], antigens expressed at higher levels compared to normal cells (such as Her2/neu) [9], and antigens derived from cancer-inducing viruses (such as human papillomavirus E6 and E7 oncogene products) [10].
While the functional relevance of tumor antigen-directed immune reactions has been predominantly discussed for the cellular part of the immune system [11], antibodies raised against tumor antigens have been primarily used to indirectly profile the expression of the antigens [12]. This has led to the discovery of previously unknown tumor antigens (e.g., NY-ESO1) by screening expression libraries for antibody-reactive epitopes (SEREX approach) [13]. It has also been attempted to identify antigen panels against which serum antibody detection may have diagnostic importance as early cancer detection markers or test for recurrence [12, 14]. Data on antibody levels in precancerous stages are scarce, and antibody prevalence for most tumor antigens in cancer patients is low, precluding use in diagnostic assays for early cancer detection (median antibody frequency was 14 % in a systematic review) [12]. There is, however, some promising data on increasing sensitivity for cancer detection using panels of antigens [14].
For high-throughput serologic testing against conventional antigens in addition to arrays [15], various bead-based multiplex assays have been described [16]. In bead-based assays, the antigens are classically linked to different microspheres that can be mixed to test for serum antibodies in a multiplex approach. During subsequent readout in a FACS-like LASER device, differentiation of individual antigen-reactive antibodies is guaranteed by different internal fluorescence signals of the different antigen-coated bead types. In parallel, quantification of a second fluorescence signal indicates antibody binding [16].
Driven by advances in whole genome and exome sequencing combined with computational cancer research, the repertoire of cancer-related mutant genes potentially translated into immunogenic tumor neo-antigens is rapidly increasing [17]. This generates the need for novel high-throughput technologies that allow profiling antibody patterns against a large number of predicted antigens in large sets of sera. In addition to study diagnostic applications of humoral immune responses against neo-antigens, individualized therapeutic vaccination from the large repertoire of cancer mutation-derived antigens is being considered [18] and assays are being needed for immune monitoring.
We here describe a bead-based multiplex assay to test for humoral immune responses against linear epitopes from a large panel of in silico-predicted tumor antigens. Our methodic approach includes over 30 synthetic biotinylated peptides comprising the predicted neo-antigens. They are fused to a FLAG epitope to ensure monitoring antigen binding to avidin-linked microspheres in the absence of monoclonal antibodies against the large number of predicted antigenic epitopes. The predicted tumor antigens that were used to establish the protocol are derived from translational products of known frameshift mutations in coding microsatellites characteristic of microsatellite-unstable colorectal cancer [19]. Against few of these antigens, humoral immune responses had been confirmed previously in patients with microsatellite-unstable colorectal cancer using peptide ELISA [20] supporting the significance of the model concept. The described method can be translated to other concepts representing a general approach to screen for antibodies using synthetic linear peptide epitopes.
Materials and methods
Peptides
Amino acid sequences have been computed for 32 peptides derived from mutated (−1 and −2 shift) microsatellite-containing genes with a published mutation frequency in microsatellite-unstable colorectal cancer of over 60 % (www.seltarbase.org) (Supplemental Table). High-grade-synthetic HPLC-purified peptides with >95 % purity with the following specifications were used: N-terminal biotin and a 6-aminohexanoic acid (Ahx) spacer, followed by the antigenic amino acid sequence and a C-terminal FLAG octapeptide (Genaxxon Bioscience). A peptide lacking antigenic amino acids consisting of the biotin, Ahx spacer, and the FLAG epitope only was used as control. Peptides were solubilized in dimethylsulfoxid (DMSO) at 5 mg/ml stocks and stored at −80 °C.
Coupling of peptides to avidin-polystyrene beads
Polystyrene microspheres internally labeled with fluorescent dyes and containing surface avidin groups for near covalent binding to the biotinylated peptides were provided at 2.5 million beads per ml in a phosphate buffer solution containing BSA, Tween 20, and sodium azide as a preservative (LumAvidin, Luminex Corp., Austin, TX). The desired number of beads was withdrawn, spun down at 13,000 rpm, and washed in PBS plus 0.1 % casein. Prior to dispensing, it was always ensured that microspheres were homogeneously resuspended. Biotinylated peptides were diluted from DMSO stocks in PBS plus 0.1 % casein to reach the desired peptide concentration. They were coupled to individual fluorescent microspheres to allow for differentiation of the response to the different peptide antigens in the multiplex assay by differentiation of the different fluorescent dyes. The washed bead pellet was sonicated and resuspended in the peptide dilution and incubated for 30 min on a shaker protected from light. After spinning down, the beads were washed three times in PBS plus 0.1 % casein and incubated for 30 min in 1 µM biotin (Sigma-Aldrich) to block residual-free avidin. After an additional washing step, peptide-coated beads were resuspended in storage buffer containing PBS, 0.1 % casein, and 0.05 % sodium azide and stored at 4 °C protected from light until use.
Confirmation of peptide coupling
Successful coupling of the biotinylated peptides to the microspheres was monitored by determining anti-FLAG reactivity. This was done in single-plex measurements of individual bead types as well as in multiplexed detection in the microsphere mix. For each bead type, 3,000 beads coupled with the different peptides were used, mixed in case of multiplex detection and incubated with a monoclonal anti-FLAG antibody (M2, Sigma; 1:1,000) in 96-well filter bottom plates (Millipore) for 1 h at room temperature on a shaker. After washing in PBS, 0.1 % casein on a vacuum pump (Millipore), a phycoerythrin-labeled anti-mouse IgG antibody (goat Anti-Mouse IgG (H+L)–PE, Molecular Probes; 1:2,000) was incubated for 30 min. After an additional washing step, beads were resuspended in the filter plate in PBS, 0.1 % casein and fluorescence was measured with the Luminex 100™ system.
Peptide coupling was additionally evaluated indirectly by the determination of free avidin on peptide-coupled beads. Therefore, peptide-coupled beads were incubated for 30 min with a biotinylated antibody (mouse anti-rabbit light chain-specific biotin-conjugated monoclonal antibody, Millipore, 1:1,000). Binding of this antibody to free avidin was determined by a phycoerythrin-labeled anti-mouse IgG antibody (goat Anti-Mouse IgG (H+L)–PE, Molecular Probes; 1:2,000) and readout in the Luminex 100™ system.
Serum testing
Sera were preincubated at a dilution of 1:50 in 0.5 % casein-PBS blocking buffer containing 0.5 % polyvinylalcohol, 0.8 % polyvinylpyrrolidone, and 2.5 % CBS-K to suppress non-specific binding of sera to the microspheres (according to Waterboer et al. [21]) and afterward diluted to a final concentration of 1:100 with the peptide-coupled microsphere mix (3,000 beads per bead type) in filter plates. Signal detection was done using a PE-labeled anti-human IgG antibody (goat anti-human IgG (H+L)–PE, Jackson Immune Research, 1:150) for human sera or PE-labeled anti-rabbit Ig antibody (R-phycoerythrin F(ab’)2 Fragment of Goat Anti-Rabbit IgG (H+L)) for rabbit sera. Readout of median fluorescence intensity (MFI) was done with the Luminex 100™ system.
Origin of rabbit and human sera
The analytical performance of the assay was evaluated with rabbit and human sera with known antibody status against distinct antigens included in the assay. Rabbit sera were derived from rabbits either immunized with neo-peptide sequences included in the assay (ACVR2(-1) or TGFBR2(-1)) or not included in the assay (AIM2(-1)) (Charles River, Kisslegg, Germany). Human sera were derived from patients with microsatellite-unstable colorectal cancer before surgery and from one patient with microsatellite-unstable colorectal cancer immunized with two neo-peptide sequences included in the assay (TAF1B(-1) and HT001(-1)), and one peptide not included in the assay (AIM2(-1)) in a therapeutic vaccine trial (Micoryx Trial, ClinicalTrials.gov Identifier: NCT01461148, Sponsor: Oryx GmbH und Co KG, Marktplatz 1, 85598 Baldham, Germany).
Peptide ELISA
Serum reactivity in the multiplex assay was compared with peptide ELISA. Peptide ELISA was performed as published previously [20]. Peptides were coated to 96-well polystyrol microtiter plates “Maxisorp” (Nunc, Roskilde, Denmark) at a concentration of 40 µg/ml in PBS. Peptide binding to the microtiter plates and optimal saturating peptide concentration were assessed using an alkaline phosphatase—peptide competition assay according to a previously published protocol [22]. Sera were diluted 1:100 in PBS, 0.5 % casein, and antibodies detected with a HRP-labeled rabbit anti-human-IgG antibody (Jackson Immunoresearch, West Grove, PA; 1:10,000 in PBS, 0.5 % casein) and TMB substrate (Sigma, Deisenhofen, Germany). Optical density was measured at 450 nm after addition of 1 N H2SO4.
Results
Binding efficiency of biotinylated peptides to avidin-polystyrene beads
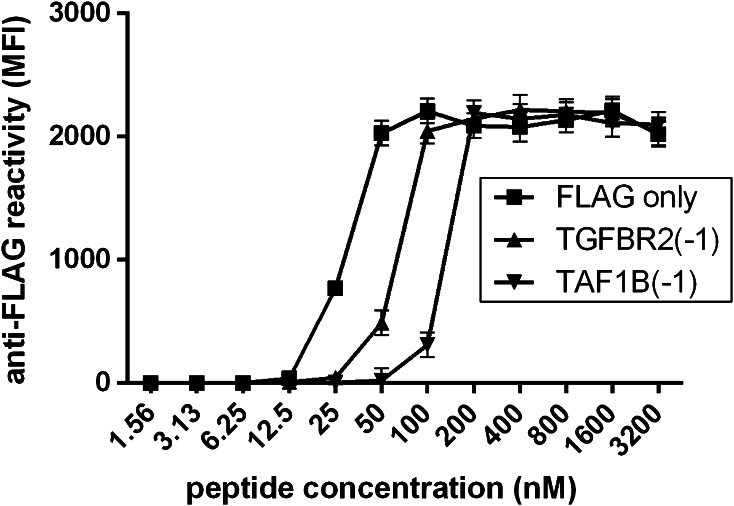
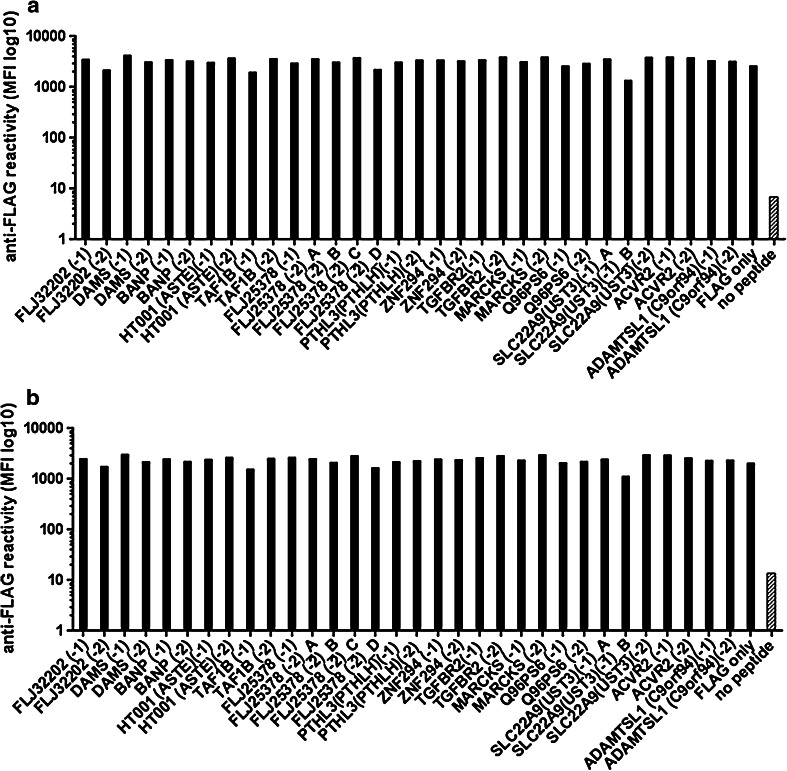
For a total of 32 in silico-predicted antigens, the respective biotinylated FLAG-tagged peptides have been synthesized (Supplemental Table) and the multiplex assay has been established and validated. In a first step, the peptide concentration required to yield maximum signal intensity with anti-FLAG antibody detection (Fig. 1a) was determined. This was done by extensive serial dilutions of three peptides from 3.20 μM to 1.56 nM in single-plex approaches. The signal of anti-FLAG antibody reactivity reached a maximum for all three peptides at a peptide concentration between 50 and 200 nM (Fig. 2). Higher peptide concentrations did not increase signal intensity, and lower peptide concentrations substantially decreased the signal (Fig. 2). Based on this finding, a peptide concentration of 400 nM was assumed sufficient to yield saturation of the avidin groups of the beads and was subsequently evaluated for all 32 peptides. Signal intensity of anti-FLAG antibody detection was in the same range for all 32 peptides in single-plex approaches, confirming successful binding of all peptides to the individual bead types at the 400-nM concentration (Fig. 3a).
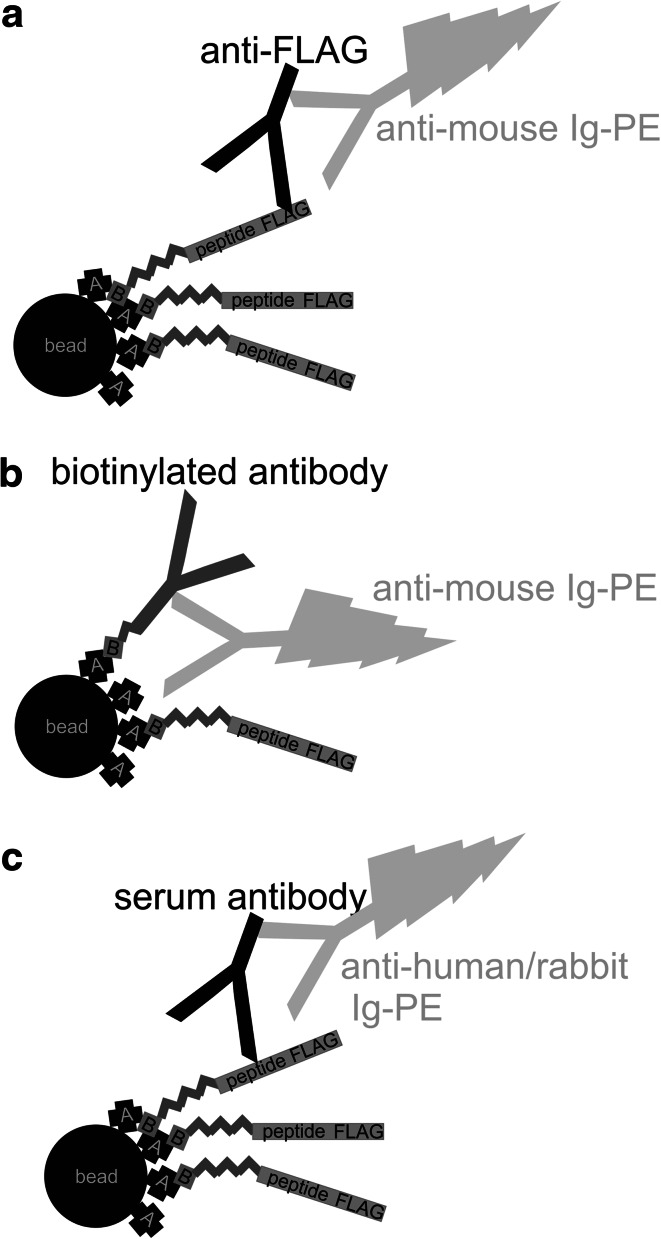
Fig. 1.
Schematic illustration of the methodic approach. a Validation of peptide binding to the beads by anti-FLAG reactivity. b Determination of free avidin by quantification using a biotinylated antibody. c Detection of serum antibodies
Fig. 2.
Determination of saturating peptide concentration. Shown is the anti-FLAG reactivity (Y axis) in beads coupled with peptide concentrations from 1.56 nM to 3200 nM (X axis) for three peptides (FLAG only, TGFBR2(-1), and TAF1B(-1))
Fig. 3.
Confirmation of peptide binding to the beads. a High anti-FLAG reactivity for single-plex signals of the different FLAG-peptide-coupled beads and low reactivity for empty beads (gray bar), indicating successful binding of FLAG-peptides and specificity of the anti-FLAG signal. b High anti-FLAG reactivity in the multi-plex approach for all FLAG-peptide-coupled beads and still low reactivity for empty beads, indicating no detectable carryover of peptides to the empty beads supporting successful peptide binding without significant dissociation of peptides from the beads
Next, non-specific carryover of free unbound peptides to unintended bead types during mixing of the peptide-coupled beads in the multiplex approach was evaluated. Beads were coupled with the different peptides at a concentration of 400 nM, mixed together with peptide-empty avidin beads, incubated over night to allow possible dissociation of peptides and re-coupling. Subsequently, signal intensity with anti-FLAG antibody detection was measured for all bead types in a multiplex approach. In order to block residual-free avidin groups after peptide coupling, the beads were incubated in biotin-containing buffer before mixing. Signal detection by an anti-FLAG antibody did confirm strong signals for all peptide-bound bead types (Fig. 3b). Low signal was detected for the peptide-empty bead type compatible with sole auto-fluorescence, indicating no carryover of peptides to the empty beads (Fig. 3b). The same approach without the biotin-blocking step indicated signals in the empty beads similar to the ones observed for the peptide-bound beads. This indicated carryover and binding of FLAG-tagged peptides after mixing and supported the importance of the biotin-blocking step.
Finally, we additionally excluded the presence of a significant amount of free avidin on the beads after peptide coupling. This allowed to prove saturation of the beads with the biotinylated peptides and to further exclude possible binding of free peptides to free avidin after mixing the beads in the multiplex approach. The different peptides-coupled beads were mixed together with empty, non-biotin/peptide-bound avidin beads and processed with and without the biotin-blocking procedure according to the protocol. Empty avidin was detected by a biotinylated antibody (Fig. 1b). Low signals were observed for all beads treated according to the protocol, indicating no significant free avidin. Strong signals were observed for single bead types, and the peptide-empty beads in line with the expected free avidin and supporting the importance of the blocking step (data not shown).
Analytical specificity of the assay
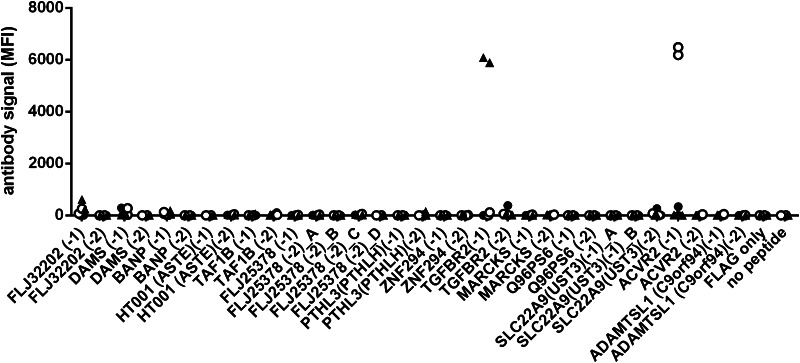
After successful coupling of the peptides to the beads and confirmation of no significant dissociation and carryover in the multiplex approach, analytical specificity of the assay was assessed. Therefore, peptide-reactive antibodies were measured in sera from rabbits immunized with peptides included in the assay (ACVR2(-1) or TGFBR2(-1)). As a control, sera from rabbits immunized with a peptide antigen not included in the assay (AIM2(-1)) were examined. Two rabbits immunized with the control peptide AIM2(-1) showed low reactivity against all peptides of the assay (median MFI of all bead types is 0 for both rabbits, range between 0 and 381). In contrast, four rabbits immunized with ACVR2(-1) or TGFBR2(-1) had strong antibody signals against the peptides used for immunization (MFI 6181 and 6481 for ACVR2(-1) and MFI 6081 and 5887 for TGFBR2(-1), Fig. 4).
Fig. 4.
Serum reactivity in the multiplex approach of rabbits immunized with AIM2(-1) (rabbit#1, rabbit#2, indicated by filled dots), immunized with ACVR2(-1) (rabbit#3, rabbit#4, indicated by open circles), and TGFBR2(1) (rabbit#5, rabbit#6, indicated by filled triangles). The AIM2(-1) peptide is not included in the assay, and accordingly, rabbit#1 and rabbit#2 (filled dots) show low reactivity against all peptides of the assay. Rabbit#3 and rabbit#4 (open circles) have strong antibody signals against ACVR2(-1), and rabbit#5 and rabbit#6 (filled triangles) have strong antibody signals against TGFBR2(-1), corresponding to the peptides with which they have been immunized
Human serum reactivity
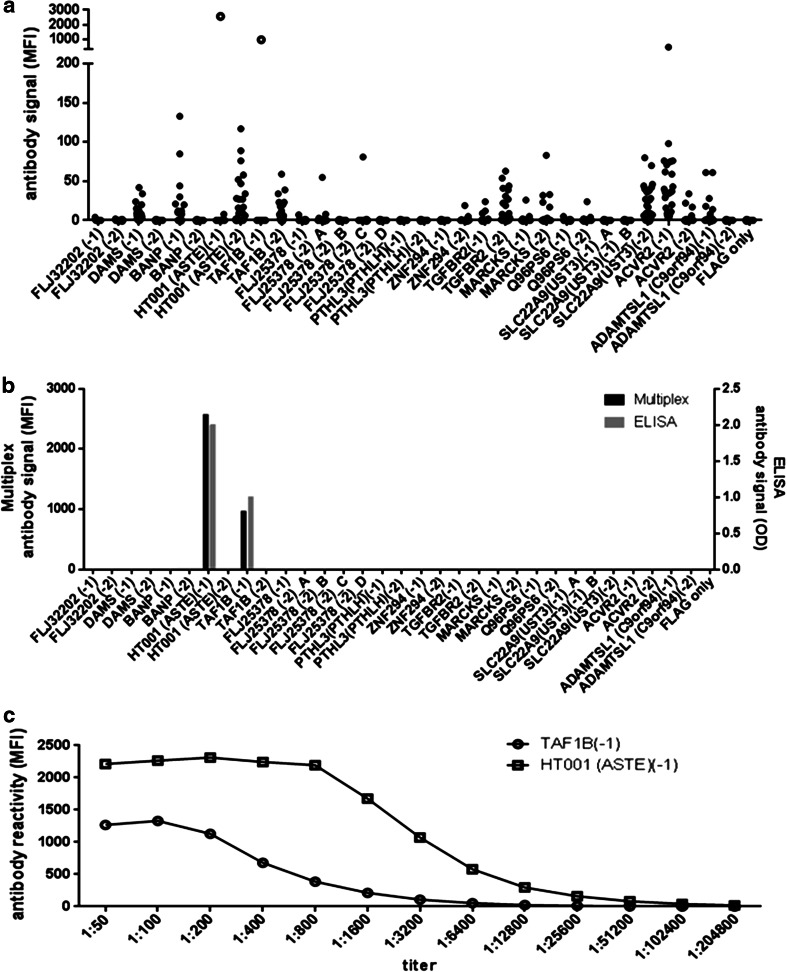
With the established protocol, human sera were tested in the multiplex approach according to a previously published protocol for reduced non-specific serum reactivity in bead-based assays [21]. Median fluorescence intensity signals were background-corrected by subtracting FLAG-only-peptide reactions. Net reactions against various antigens could be observed in single sera from patients with microsatellite-unstable colorectal cancer, suggesting antigen-specific antibodies (Fig. 5a). To further prove specificity of the signals, one serum from a patient vaccinated in a clinical trial (Micoryx, ClinicalTrials.gov Identifier: NCT01461148) with two of the peptides included in the assay was analyzed. This serum showed highest reactivity against the vaccine peptides (Fig. 5a, open circles). The same serum was tested in a peptide ELISA against all peptides included in the multiplex assay, and reactivity was compared to the multiplex assay. Complete agreement was seen, with strong signals against two peptides and no reactivity against the remaining peptides (Fig. 5b). In order to estimate the detection limit of the assay, the vaccinated patient’s serum was titrated up to 1:204,800. Net reactivity against the two vaccine peptides was detectable with a maximum serum dilution of 1:3,200 against TAF1B(-1) and even 1:51,200 against HT001(ASTE)(-1) (Fig. 5c), indicating high analytical sensitivity.
Fig. 5.
Human serum reactivity against all peptides. a Background-normalized netto reactivity (MFI) in the multiplex assay of 20 sera from patients with microsatellite-unstable colorectal cancer (filled dots) and of one serum from a patient with microsatellite-unstable colorectal cancer vaccinated with HT001(-1), TAF1B(-1), and AIM2(-1) in a clinical trial (Micoryx). The latter serum (open circles) shows strong signals against the vaccine peptides included in the assay (HT001(-1) and TAF1B(-1). b Agreement between multiplex signal and ELISA signal for all peptides measured in one serum. c Titration of the serum from the vaccinated patient. Shown is the reactivity of antibodies against HT001(-1), and TAF1B(-1)
Reproducibility of the assay
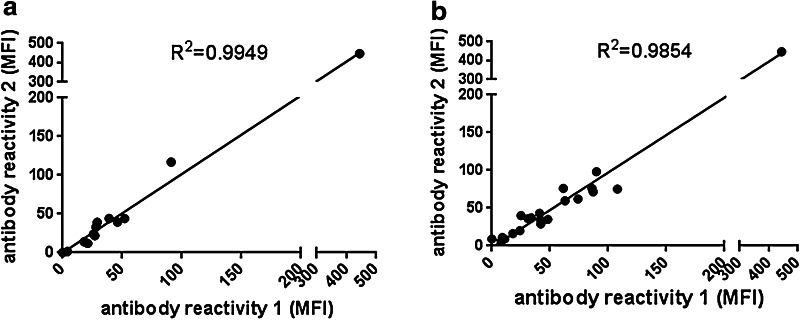
Reproducibility of serological results was assessed by inter-assay agreement of repeated multiplex testings. Reproducibility for 20 sera tested twice against all antigens was high (R 2 > 0.98 for all sera and all antigens). Figure 6 shows results for all sera against the AVCR2(-1) peptide (R 2 = 0.9949) and for one serum against all peptides (R 2 = 0.9854). The agreement is visualized for all sera and all antigens in Supplemental Figure.
Fig. 6.
Reproducibility of the multiplex assay. a Twenty sera tested twice in the multiplex assay. Shown is the reproducibility for AVCR2(-1) with each dot representing one serum. b Reproducibility of one serum against all peptides with each peptide represented by one dot
Discussion
We here describe a novel method for multiplexed detection of antibodies against a large set of in silico-predicted linear epitopes. As a model system, we used frameshift peptide antigens generated in microsatellite-unstable cancers. We coupled synthetic biotinylated peptides containing the neo-antigen sequence and a FLAG-tag to avidin-carrying fluorescent microspheres and measured antibodies using the Luminex technology. The approach allowed for monitoring of successful peptide coupling by anti-FLAG reactivity. Excellent analytical specificity of measured serum antibody reactivity was proven by the detection of vaccine-induced immune responses against distinct antigens in rabbits and in a patient with microsatellite-unstable colorectal cancer. The measured antibody responses were comparable to peptide ELISA, and inter-assay reproducibility of the multiplex approach was excellent.
Bead-based multiplex assays are advantageous compared to ELISA, if the number of antigens of interest is high and testing of a large number of sera is planned. Measuring antibodies against 50 antigens in 500 serum samples needs already 25,000 ELISA wells, ending up in more than 260 plates, without considering duplicate measurements. In a multiplex assay, each serum only requires one well containing beads with all antigens of interest. This allows testing 500 sera on only six plates. In addition to saved working load and time, the reduction of the required plates to a number that allows a minimum of separate runs may be particularly important to avoid variability in conditions between several runs.
Previous serological bead-based assays have been designed using recombinant proteins [23–25] or synthetic peptides [26–29] derived from known antigens as conformational or linear epitope structures coupled either directly via carboxyl groups [28, 30] or via avidin–biotin interactions [26, 31] to the microspheres. Detection of antibodies against 2 to 15 antigens in parallel is reported in most papers, and single approaches include over 20 [23] and 30 [32] previously characterized antigens. Most importantly, before serum testing, successful coupling of the antigens to the microspheres needs to be ensured by monoclonal antibodies against the antigens. However, the availability of monoclonal antibodies is a limitation, if not predefined antigens, but novel in silico-predicted epitopes are used. Advances in whole genome and exome sequencing and computational cancer research steadily increase the repertoire of cancer mutations potentially translated into immunogenic tumor neo-antigens [17]. Profiling antibody patterns against predicted antigens may provide novel options for cancer early detection and monitoring of cancer patients during therapy and follow-up on the basis of humoral immune responses. The addition of a FLAG epitope to the peptides in our assay allows for monitoring of successful peptide coupling by anti-FLAG antibodies. Furthermore, mutation-derived predicted neo-antigens are considered a source for individualized cancer vaccines [18] and flexible assays are important for immune monitoring on an individualized basis.
An important point central to all serologic assays will be the definition of a cutoff for positive serum responses. We intentionally decided to not present a cutoff that discriminates healthy individuals from patients with microsatellite-unstable cancers used in our model system. This would address the diagnostic sensitivity and specificity of the assay for a certain condition (microsatellite-unstable cancers), which was not the aim of the present manuscript.
We had previously published that antibodies against frameshift peptide antigens contained in the presented assay may be occasionally detected in healthy donors [20]. These data predict that the biological mechanisms underlying the formation of such antigens and immune responses against them are complex and not yet sufficiently understood. Therefore, any studies aiming at the evaluation of a potential suitability of frameshift peptide antibody responses as a biomarker should be based on a large set of prospectively collected serum samples from individuals affected by different disease conditions (e.g., Lynch syndrome mutation carriers without cancer history, those with cancer history, and sporadic microsatellite-unstable cancer patients) and healthy donors. Moreover, a very robust and reliable test format should be applied to address these questions, underlining the necessity of the development of the method described in our present study.
In conclusion, the presented methodic approach represents a high-throughput platform to profiling humoral immune responses against a large set of predicted antigens and can easily be converted from the used frameshift peptide antigens to other linear epitopes, extending the relevance of the approach as a general tool to be applied for immune monitoring and profiling approaches.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the sponsor of the Micoryx trial (ClinicalTrials.gov Identifier: NCT01461148) Oryx GmbH und Co KG, Marktplatz 1, 85598 Baldham, Germany, namely Dr. Dr. Michael W. Dahm, Dr. Ottheinz Krebs, and Dr. Bernard Huber. This work was funded by a Grant No. 109477 from the German Cancer Aid (Deutsche Krebshilfe).
Conflict of interest
None of the authors declares a conflict of interest.
References
- 1.Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Liefeldt L, Brakemeier S, Glander P, Waiser J, Lachmann N, Schonemann C, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant. 2012;12:1192–1198. doi: 10.1111/j.1600-6143.2011.03961.x. [DOI] [PubMed] [Google Scholar]
- 3.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca C, Dranoff G. Capitalizing on the immunogenicity of dying tumor cells. Clin Cancer Res. 2008;14:1603–1608. doi: 10.1158/1078-0432.CCR-07-2245. [DOI] [PubMed] [Google Scholar]
- 5.Houghton AN. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 7.Jäger E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jäger D, et al. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(SICI)1097-0215(19991022)84:5<506::AID-IJC10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Graves CR, Robertson JF, Murray A, Price MR, Chapman CJ. Malignancy-induced autoimmunity to MUC1: initial antibody characterization. J Pept Res. 2005;66:357–363. doi: 10.1111/j.1399-3011.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 9.Traina A, Agostara B, Marasa L, Calabro M, Zarcone M, Carruba G. HER2/neu expression in relation to clinicopathologic features of breast cancer patients. Ann N Y Acad Sci. 2006;1089:159–167. doi: 10.1196/annals.1386.029. [DOI] [PubMed] [Google Scholar]
- 10.Reuschenbach M, Waterboer T, Wallin KL, Einenkel J, Dillner J, Hamsikova E, et al. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer. 2008;123:2626–2631. doi: 10.1002/ijc.23837. [DOI] [PubMed] [Google Scholar]
- 11.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 12.Reuschenbach M, von Knebel DM, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G, Miles A, Line A, Rees RC. Identification of tumour antigens by serological analysis of cDNA expression cloning. Cancer Immunol Immunother. 2004;53:139–143. doi: 10.1007/s00262-003-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–1394. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 15.Gnjatic S, Wheeler C, Ebner M, Ritter E, Murray A, Altorki NK, et al. Seromic analysis of antibody responses in non-small cell lung cancer patients and healthy donors using conformational protein arrays. J Immunol Methods. 2009;341:50–58. doi: 10.1016/j.jim.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Binder SR. Autoantibody detection using multiplex technologies. Lupus. 2006;15:412–421. doi: 10.1191/0961203306lu2326oa. [DOI] [PubMed] [Google Scholar]
- 17.Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet. 2013;14:703–718. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castle JC, Kreiter S, Diekmann J, Lower M, van de Roemer N, de Graaf J, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72:1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 19.Banerjea A, Bustin SA, Dorudi S. The immunogenicity of colorectal cancers with high-degree microsatellite instability. World J Surg Oncol. 2005;3:26. doi: 10.1186/1477-7819-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuschenbach M, Kloor M, Morak M, Wentzensen N, Germann A, Garbe Y, et al. Serum antibodies against frameshift peptides in microsatellite unstable colorectal cancer patients with Lynch syndrome. Fam Cancer. 2010;9:173–179. doi: 10.1007/s10689-009-9307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309:200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Steinitz M, Baraz L. A rapid method for estimating the binding of ligands to ELISA microwells. J Immunol Methods. 2000;238:143–150. doi: 10.1016/S0022-1759(00)00160-5. [DOI] [PubMed] [Google Scholar]
- 23.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 24.Jones LP, Zheng HQ, Karron RA, Peret TC, Tsou C, Anderson LJ. Multiplex assay for detection of strain-specific antibodies against the two variable regions of the G protein of respiratory syncytial virus. Clin Diagn Lab Immunol. 2002;9:633–638. doi: 10.1128/CDLI.9.3.633-638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drummond JE (2009) Multiplexed analysis of peptide antigen-specific antibodies. Curr Protoc Cytom Chapter 13:Unit 13.10. doi:10.1002/0471142956.cy1310s48 [DOI] [PubMed]
- 27.Drummond JE, Shaw EE, Antonello JM, Green T, Page GJ, Motley CO, et al. Design and optimization of a multiplex anti-influenza peptide immunoassay. J Immunol Methods. 2008;334:11–20. doi: 10.1016/j.jim.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosino E, Dumoulin C, Orlandi-Pradines E, Remoue F, Toure-Balde A, Tall A, et al. A multiplex assay for the simultaneous detection of antibodies against 15 Plasmodium falciparum and Anopheles gambiae saliva antigens. Malar J. 2010;9:317. doi: 10.1186/1475-2875-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brink M, Hansson M, Mathsson L, Jakobsson PJ, Holmdahl R, Hallmans G, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013;65:899–910. doi: 10.1002/art.37835. [DOI] [PubMed] [Google Scholar]
- 30.Nogueira RD, King WF, Gunda G, Culshaw S, Taubman MA, Mattos-Graner RO, et al. Mutans streptococcal infection induces salivary antibody to virulence proteins and associated functional domains. Infect Immun. 2008;76:3606–3613. doi: 10.1128/IAI.00214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong W, Metcalfe P, Mushens R, Lucas G, Ouwehand WH, Navarrete CV. Detection of human platelet antigen-1a alloantibodies in cases of fetomaternal alloimmune thrombocytopenia using recombinant beta3 integrin fragments coupled to fluorescently labeled beads. Transfusion. 2011;51:1261–1270. doi: 10.1111/j.1537-2995.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 32.Waterboer T, Abeni D, Sampogna F, Rother A, Masini C, Sehr P, et al. Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. Br J Dermatol. 2008;159:457–459. doi: 10.1111/j.1365-2133.2008.08621.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.