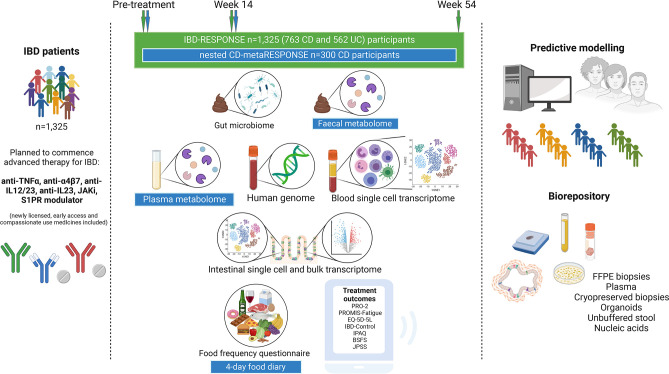
Figure 2.
Study overview schematic. 1325 participants with IBD planned to commence an advanced therapy will be recruited, including a nested subcohort of 300 patients with CD (CD-metaRESPONSE). All participants will collect two stool sample tubes at each study assessment timepoint (baseline, week 14 and week 54). CD-metaRESPONSE participants will be required to collect a third stool sample tube at baseline and week 14. If a participant attends hospital for a face-to-face appointment within the baseline and/or week 14 study assessment window, blood samples will be collected. If a participant attends hospital for a lower gastrointestinal endoscopy at any time during the study period (pre-treatment or post-treatment), biopsy samples will be collected. Participants will complete several questionnaires at each assessment timepoint. For CD-metaRESPONSE participants, additional detailed analyses will be undertaken of metabolic profiles (metabolome) in stool and matched blood plus in-depth dietary assessment (additional elements highlighted in blue boxes). Data generated will be used to perform predictive modelling. Any remaining participant samples will form a large biorepository for use in future research (figure created with BioRender.com). Anti-TNFα, antiumour necrosis factor alpha; anti-IL, anti-interleukin; BSFS, Bristol Stool Form Scale; CD, Crohn’s disease; FFPE, formalin-fixed paraffin embedded; IBD, inflammatory bowel disease; IPAQ, International Physical Activity Questionnaire; JAKi, Janus kinase inhibitor; JPSS, Joint Pain and Stiffness Score; PRO-2, patient-reported outcome-2; PROMIS, Patient-Reported Outcomes Measurement Information System; S1PR, sphingosine-1-phosphate receptor; UC, ulcerative colitis.