Abstract
A wide variety of cancer types has been associated with paraneoplastic autoimmune disorders and with the induction of autoimmunity against several autoantigens, among them self-antigens that are also expressed by tumor cells. This raises the question of autoimmune disorders as a result of immune reactions to the tumor. To date, however, requirements for the generation of autoimmune reactions in cancer patients remain largely unclear. In this study, we characterized conditions in altogether 131 patients, which determine autoimmune responses in primary breast cancer patients. We used ex vivo IFN-γ EliSpot assays against autologous tumor or skin lysates to evaluate tumor- and auto-reactive T-cells (TCs) in the bone marrow (BM) as well as ELISA, ECLIA, and turbidimetric immunoassays for the detection of auto-reactive antibodies in the peripheral blood and compared results to intratumoral cytokine concentrations and pathobiological features of the primary tumor tissue. We here demonstrate a significant correlation between anti-tumor BMTC responses and cellular autoimmune reactivity in primary breast cancer patients (P = 0.002). Humoral autoimmune reactions, however, were negatively correlated with anti-tumor TC immunity (P = 0.039). We observed auto-reactive BMTCs especially in patients with well-differentiated, hormone receptor-positive carcinomas (P = 0.009). Furthermore, elevated concentrations of intratumoral IFN-α significantly correlated with the induction of cellular autoimmune reactivity (P = 0.0002), while humoral autoimmune reactions correlated with increased levels of intratumoral IL-12 (P = 0.04). Altogether, these data indicate a significant role of the tumor microenvironment and particularly that of IFN-α and IL-12 in the induction of systemic autoimmune responses and imply that the primary tumor tissue represents an integral site of autoimmune regulation in cancer patients.
Keywords: Breast cancer, Autoimmunity, Tumor biology, Bone marrow, T-cell immunity
Introduction
Paraneoplastic phenomena represent a wide range of clinical syndromes that may be encountered among patients with malignant disease. About 7–10% of cancer patients develop a paraneoplastic syndrome, and a large variety of cancer types has been associated with paraneoplastic autoimmune disease [1]. Tumor-associated autoimmune disorders, therefore, represent a relevant issue in clinical practice. According to several studies, breast cancer and other malignancies are associated with the induction of autoimmunity in the form of autoantibodies against a large variety of different autoantigens [2–7], among them self-antigens that are also expressed by tumor cells, which raises the question of autoimmune disorders as a result of immune reactions to the tumor.
During the last years, evidence has accrued that malignant human tumors can be naturally recognized by the host’s immune system and induce spontaneous tumor antigen-specific T-cell (TC) responses, which together constitute an antitumoral TC memory repertoire in the bone marrow [8–12]. Approximately 30–40% of breast cancer patients develop spontaneous tumor-reactive memory TCs in their bone marrow (BM), and immune infiltrates have been detected frequently in breast cancer lesions [10, 13]. Interestingly, however, TC reactivity not only against tumor antigens (TA) but also against non-malignant breast tissue-associated antigens was found in the bone marrow of breast cancer patients [14].
In this context, we recently determined specific pathobiological features of the primary tumor tissue that are linked to spontaneous, tumor-reactive immune responses in primary breast cancer patients. Furthermore, distinct intratumoral cytokine microenvironments showed a significant impact on the induction of tumor-specific BMTC immunity [15]. To date, however, the conditions required for the generation of either humoral or cellular paraneoplastic autoimmune phenomena remain largely unclear.
In altogether 131 patients, we now demonstrate a significant correlation between anti-tumor BMTC immunity and cellular autoimmune reactivity in primary breast cancer patients, while humoral autoimmune reactions were negatively correlated with anti-tumor BMTC immunity. Strikingly, spontaneous autoimmune responses were determined by specific pathobiological features of the primary tumor in terms of good differentiation and hormone receptor expression as well as by distinct intratumoral concentrations of IFN-α and IL-12. These findings suggest a significant role of the tumor microenvironment in the induction of systemic autoimmune responses and imply that the primary tumor tissue represents an integral site of autoimmune regulation in cancer patients.
Materials and methods
Patients
BM and PB samples were taken from patients with primary, histologically approved breast carcinomas (mean age 55.4 years, range 30–80 years). Informed consent was obtained from all participants. The study protocol was approved by the ethical committee of the University of Heidelberg (Heidelberg, Germany). BM was aspirated from each anterior iliac crest during primary surgery as described [8, 16]. Heparinized BM was subjected to Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden), and cells in interphase were collected. Primary breast tumor specimens and histologically non-malignant skin samples were obtained during primary surgery and immediately snap-frozen.
Generation of DCs and T lymphocytes
Dendritic cells (DCs) were generated according to standard procedures with modifications [14]. BM cells were cultured for 14 days in serum-free X-VIVO 20 medium (BioWhittaker, Walkersville, Maryland, USA) with human GM-CSF (50 ng/ml; Behringwerke, Marburg, Germany) and IL-4 (1,000 U/ml; PromoCell, Heidelberg, Germany). Non-adherent DCs were enriched by depletion of contaminating T and B lymphocytes and pulsed for 20 h with lysates (200 μg protein/1 × 106 cells/ml) from freshly isolated autologous tumor/skin cells or normal PBMCs that were lysed by five freeze/thaw cycles [17]. To generate T lymphocytes, BM cells were incubated for 13 days in RPMI-1640 with 10% human AB serum (PromoCell), IL-2 (100 U/ml; Chiron, Ratingen, Germany), and IL-4 (60 U/ml; PromoCell) followed by overnight incubation in the same medium without interleukins. After depletion of CD19+, CD15+, and CD56+ cells, the suspension contained 95–99% CD3+ T-cells (~25% were CD8+).
IFN-γ EliSpot assays
IFN-γ-producing T lymphocytes were determined as described [10]. DCs pulsed with test or control antigens were coincubated with autologous TCs (DC/T-cell ratio, 1:5) for 40 h. The number of IFN-γ-spot-forming cells was counted using a microscope Axioplan 2 and KS ELISPOT software (Carl Zeiss Vision, Hallbergmoos, Germany). Spots measured in the presence of DCs pulsed with negative control antigens (autologous PBMC lysate) were considered as non-specific background. Individuals were designated as responders if the average number of spots in the presence of DCs loaded with test antigen (autologous tumor/skin lysate) was significantly higher (*, P < 0.05, two-sided, unpaired Student’s t test) than that in negative control wells.
Histopathologic evaluation of breast tumors
Tumors were classified with respect to the tumor-node-metastasis classification and graded according to Scarf-Bloom-Richardson [18]. Evaluation of estrogen receptor (ER) and progesterone receptor (PR) expression was performed by immunohistochemistry according to the immune-reactive score criteria. The status of receptor expression was considered negative when ER/PR expressions scored <2. Her-2-status was determined in the usual way by immunohistochemistry (IHC) and/or fluorescence in situ hybridization analysis (FISH). Her-2-status was considered positive in cases of IHC 3+ staining and/or amplification in FISH analysis, performed in cases of IHC 2+ staining.
Cytokine quantification
Primary breast tumor specimens were obtained during tumor resection and immediately snap-frozen. Specimens were mechanically homogenized and processed as described [10, 15]. Cytokines (IFN-α, TGF-β, IL-10, IL-12) were quantified by respective quantitative sandwich enzyme immunoassay technique kits according to the manufacturer’s protocol (R&D Systems, Wiesbaden, Germany). The used IFN-α detection kits comprise all subsets of IFN-α with a detection rate starting at 10 pg/ml. The standard curve for this kit was constructed with human IFN-αA (R&D Systems, Wiesbaden, Germany). Mean minimum detectable dose (MDD) of TGF-β is 4.61 pg/ml, using highly purified CHO cell-expressed recombinant human TGF-β1 for calibration. MDD of human IL-10 and IL-12 is typically less than 0.5 pg/ml. Highly purified Sf 21-expressed recombinant human IL-10 and IL-12 are used for calibration (R&D Systems, Wiesbaden, Germany). Samples have been tested in a duplicate setup. Determination of the optical density was performed on a microplate reader (Labsystems, Helsinki, Finland) according to the manufacturer’s protocol (R&D Systems, Wiesbaden, Germany).
Detection of autoantibodies
Serum samples were taken from patients with primary, histologically approved breast carcinomas. Specimens were analyzed for anti-nuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), thyroid-stimulating hormone receptor antibodies (TRAb), rheumatoid factors (RhF), and anti-thyroglobulin antibodies (TgAb) by the central laboratory of the Heidelberg University Hospital as described. ANAs and ANCAs were determined by Microtiter ELISA Kits according to the manufacturer’s protocol (Euroimmun, Lübeck, Germany). Titers less than 1:80 and 1:10, respectively, were considered within normal range. TRAbs were determined by ECLIA/Electrochemiluminescence Immunoassay (Roche Diagnostics, Mannheim, Germany) and performed on the Cobas E411 Analyzer (Roche Diagnostics, Mannheim, Germany). Concentrations less than 1 IU/l were considered within normal range. RhF were determined by Turbidimetric Immunoassay/TIA (Siemens Healthcare Diagnostics, Eschborn, Germany) and performed on the ADVIA 2400 Chemistry System (Siemens Healthcare Diagnostics, Eschborn, Germany). Concentrations less than 25 IU/ml were considered within normal range. TgAbs were determined by ECLIA/Electrochemiluminescence Immunoassay (Roche Diagnostics, Mannheim, Germany) and performed on the Cobas E411 Analyzer (Roche Diagnostics, Mannheim, Germany). Concentrations less than 60 IU/ml were considered within normal range.
Statistical evaluation
Statistical significance of differential findings between test and control groups was determined by two-sided, unpaired Student’s t-test (Figs. 1a, b, c, 4a, b) or by Fisher’s exact test (Figs. 2a, b, c, d, e, 3a, b). Differences were regarded as significant (*) if p values were <0.05.
Fig. 1.
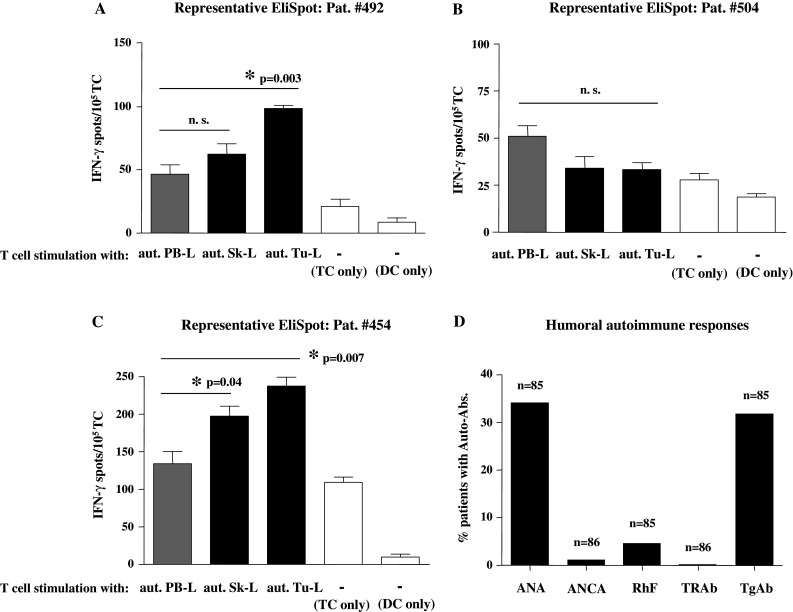
Detection of tumor-specific T-cells (TCs) and auto-reactive immune responses in primary breast cancer patients. a–c Representative results from IFN-γ EliSpot assays with BMTCs from three different individual patients tested for recognition of autologous tumor lysates (aut. Tu-L, black bars) or autologous skin lysates (aut. Sk-L, black bars) compared to respective negative control antigens (gray bars) from autologous PBMC lysate (aut. PB-L). Results demonstrate the presence (a Pat. #492) of merely tumor antigen-specific T-cells, (b Pat. #504) of neither tumor antigen nor autoantigen-specific T-cells or (c Pat. #454) of both tumor antigen and autoantigen-specific T-cells. White bars: spot numbers in wells containing unstimulated TCs or DCs alone. Bars show mean ± SEM of triplicate wells. *Significant (P < 0.05) difference to control group by two-sided Student’s t test; n.s. not significant. d Cumulative prevalence of autoantibodies (Auto-Abs.) in primary breast cancer patients. Percentile detection of anti-nuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), thyroid-stimulating hormone receptor antibodies (TRAb), rheumatoid factors (RhF), and anti-thyreoglobulin antibodies (TgAb) in the patient cohort
Fig. 4.
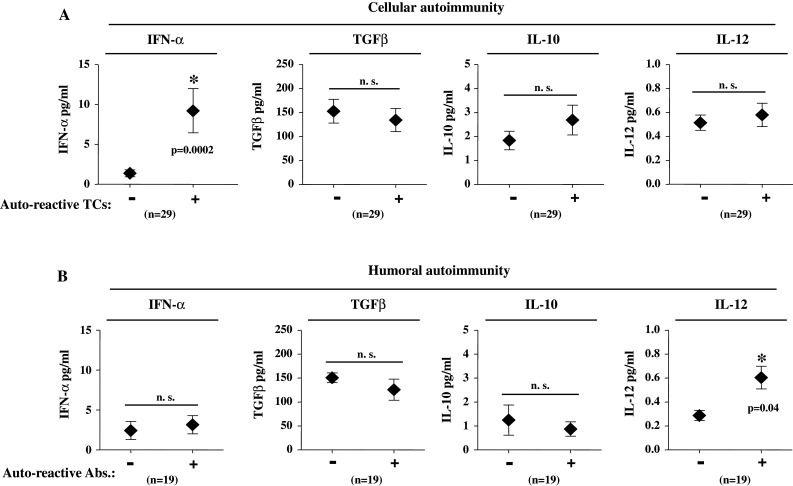
Auto-reactive immune responses in primary breast cancer patients in correlation with intratumoral cytokine concentrations. a Average concentrations of IFN-α, TGFβ, IL-10, and IL-12 in primary breast tissue. Patients are grouped according to the presence (+) or absence (−) of respective auto-reactive BMTCs. b Average concentrations of IFN-α, TGFβ, IL 10, and IL 12 in primary breast tissue. Patients are grouped according to the presence (+) or absence (−) of respective humoral autoimmunity. Presence of humoral autoimmunity was defined as at least one pathological finding within the tested antibodies (ANA, ANCA, TRAb, RhF, or TgAb). Mean ± SEM values are shown. *Significant (P < 0.05) difference between groups by two-sided Student’s t-test; n.s. not significant
Fig. 2.
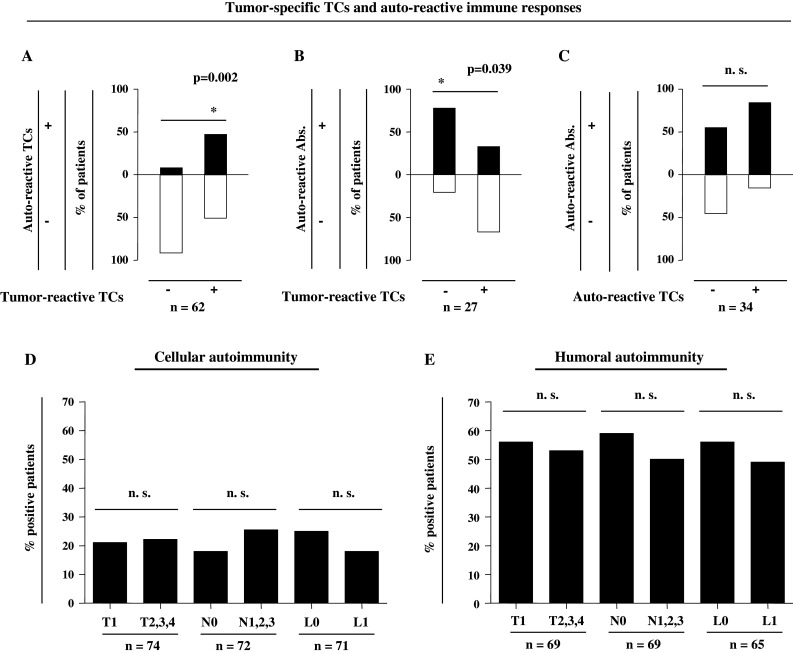
Auto-reactive immune responses in correlation with tumor-specific TCs and tumor spread. a Proportions of patients with (black bars) or without (white bars) cellular autoimmune responses according to the presence (+) or absence (−) of BMTCs reactive against autologous tumor lysates. b Proportions of patients with (black bars) or without (white bars) humoral autoimmune responses according to the presence (+) or absence (−) of BMTCs reactive against autologous tumor lysates. c Proportions of patients with (black bars) or without (white bars) humoral autoimmune responses according to the presence (+) or absence (−) of cellular autoimmune responses. d Cellular autoimmunity and e humoral autoimmunity with respect to tumor size (T), lymph-node involvement (N), and lymphatic vessel invasion (L). Humoral autoimmunity was defined as at least one pathological finding within the tested antibodies (ANA, ANCA, TRAb, RhF, or TgAb). *Significant (P < 0.05) difference by Fisher’s exact test; n.s. not significant
Fig. 3.
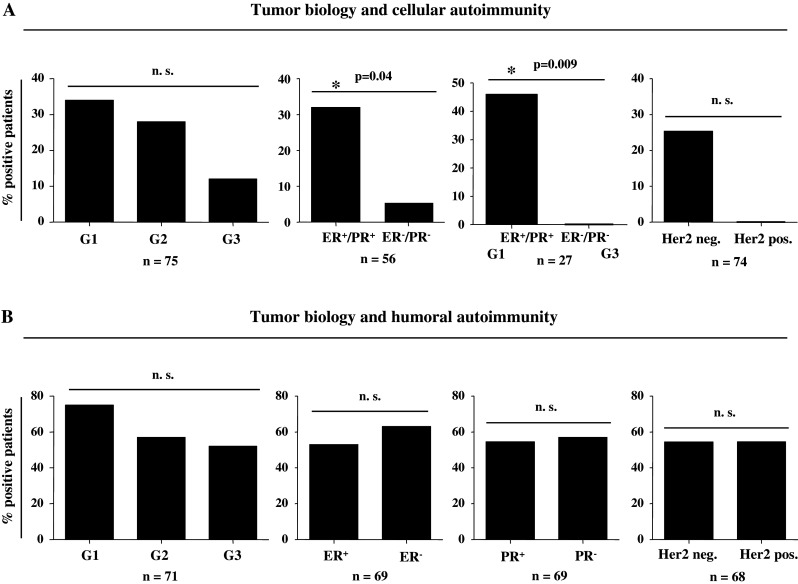
Tumor biology and auto-reactive immune responses. a Cellular autoimmunity and b humoral autoimmunity in relation to pathobiological features of the primary tumor. a Proportions of patients with auto-reactive TCs or b auto-reactive antibodies according to grading (G), hormone receptor expression (ER, PR), Her2/neu status, or stratified combinations of tumor differentiation and hormone receptor expression. Humoral autoimmunity was defined as at least one pathological finding within the tested antibodies (ANA, ANCA, TRAb, RhF, or TgAb). *Significant (P < 0.05) difference by Fisher’s exact test; n.s. not significant
Results
Into this study, we included a total of 131 primary, non-metastasized patients with histologically approved breast cancer. Patients with in situ disease only were excluded. Bone marrow, peripheral blood as well as tumor and skin specimens were obtained during primary tumor resection.
Spontaneous tumor- and auto-reactive T-cell responses in breast cancer patients
We performed short-term IFN-γ EliSpot assays with unstimulated T-cells and antigen-presenting autologous DCs from the BM of altogether 81 primarily operated breast cancer patients to test for the presence of functional, tumor- or autoantigen-specific T-cells as secondary immune responses. Autologous DCs loaded with respective antigens were used for restimulation of ex vivo isolated BMTCs during 40-h EliSpot assays. Antigen-specific IFN-γ is exclusively secreted by pre-existing memory T-cells [19, 20]. Unspecific background reactivity can be determined by stimulation of TCs with irrelevant control antigens. To assess a broad repertoire of tumor- or auto-reactive CD8+ and CD4+ T-cells, we tested TC reactivity against autologous DCs pulsed with autologous tumor cell lysates or lysates of autologous, histologically non-malignant skin specimens as a source of autoantigens. DCs pulsed with lysates of autologous PBMCs (PB-L) were used as negative controls.
In Fig. 1a–c, representative IFN-γ EliSpot data are shown. Antigen-specific responses are defined by a significantly increased spot number in triplicate test wells compared to corresponding control wells and indicated by asterisks. Figure 1a depicts a representative patient (Pat. #492) with TA-specific TCs (Tu-L), while auto-reactive TCs (Sk-L) were not observed. Alternatively, Fig. 1b shows a representative patient (Pat. #504) in whom neither tumor-reactive nor auto-reactive TCs were detected, while in Pat. #454 (Fig. 1c) both tumor-reactive and auto-reactive TCs of the BM were observed in the IFN-γ EliSpot analysis. Overall, we found tumor-reactive BMTCs in 19/64 patients (29.7%) and auto-reactive BMTCs in 17/79 patients (21.5%).
Humoral autoimmune reactivity in breast cancer patients
To also explore humoral autoimmune reactivity in primary breast cancer, we next analyzed a total of 86 patients for the presence of auto-reactive antibodies. For this reason, serum samples from breast cancer patients were taken during primary surgery and assessed for anti-nuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), thyroid-stimulating hormone receptor antibodies (TRAb), rheumatoid factors (RhF), and anti-thyreoglobulin antibodies (TgAb). In Fig. 1d, cumulative, percentile results are shown. ANAs were detected in 29/85 (34.1%), ANCAs in 3/86 (3.5%), TRAbs in 0/86 (0%), RhF in 4/85 (4.7%), and TgAbs in 27/85 (31.8%) patients analyzed. Taken together, 49 out of 86 patients (57%) showed at least one pathological result in the autoantibody tests performed and were therefore considered positive for humoral autoimmune reactions in the following analyses.
Correlation of autoimmune responses and tumor-specific TCs
We next analyzed the presence of auto-reactive BMTCs with respect to tumor-specific BMTCs. Fig. 2a shows a positive correlation of tumor-specific and cellular autoimmune responses in a total of 62 patients, with a significantly higher detection of auto-reactive BMTCs in patients with cellular anti-tumor immunity in the IFN-γ EliSpot assay (P = 0.002). Subsequently, in patients with tumor-reactive BMTCs, we detected a significantly decreased percentage of humoral autoimmune responses compared to patients without tumor-reactive cellular immune responses (Fig. 2b; n = 27; P = 0.039), thus showing a significantly negative correlation. Interestingly, however, patients with cellular autoimmune reactivity did not exhibit any significant correlation with patients with humoral autoimmunity (n = 34; Fig. 2c).
Autoimmune reactions are not influenced by local tumor spread
To clarify reasons for the induction of either cellular or humoral reactivity in primary breast cancer patients, we correlated the presence of auto-reactive BMTCs and the occurrence of autoantibodies with histopathological parameters of local tumor extent. In this analysis, we focused on tumor size (T), lymph-node involvement (N), and lymphatic vessel invasion (L). Figures 2d, e show, however, no significant correlation of the analyzed parameters with either humoral or cellular autoimmune responses.
Correlation of tumor biology and autoimmune responses
In the next analysis, we considered primary tumor differentiation (G), hormone receptor expression (ER, PR), Her2/neu status or stratified combinations of tumor differentiation and hormone receptor expression. Tumor differentiation in 75 patients was correlated with the presence of auto-reactive BMTCs in the IFN-γ EliSpot assay. By trend (Fig. 3a; P = 0.096), we observed a higher prevalence of auto-reactive BMTCs in patients with good or moderate tumor differentiation (G1, G2) compared to patients with low tumor differentiation (G3). With respect to hormone receptor expression, a significantly higher proportion (Fig. 3a; P = 0.04) of cellular autoimmune responses was detected in patients with positive hormone receptor expression (ER+, PR+) compared to hormone receptor-negative patients (ER−, PR−). In patients with a combination of good tumor differentiation (G1) and positive hormone receptor expression, we observed an even more significant difference regarding cellular autoimmune reactivity in comparison with patients with low tumor differentiation and negative hormone receptor expression (Fig. 3a; P = 0.009). Concerning the Her2/neu status, we did not see a significant difference between groups, although patients without Her2/neu expression showed a higher prevalence of cellular autoimmune responses by trend (Fig. 3a; n = 74).
Subsequently, pathobiological tumor features were analyzed with respect to humoral autoimmune reactivity in a total of 71 patients. Interestingly, however, we did not detect any significant differences in the prevalence of humoral autoimmune responses regarding tumor differentiation, hormone receptor expression, and Her2/neu status (Fig. 3b).
Determination of autoimmune reactivity by intratumoral cytokines
To further identify potential reasons for the induction of either cellular or humoral autoimmune responses in breast cancer patients, we analyzed primary tumor tissue for specific, intratumoral cytokine patterns. In this context, we chose IL-10 and IL-12 as essential regulators of autoimmune responses [21] as well as IFN-α and TGF-β due to their significant impact on BMTC immune regulation [15].
We first correlated intratumoral cytokine concentrations with auto-reactive BMTCs in the IFN-γ EliSpot assay. In patients with auto-reactive BMTCs, no significant differences were detected in the concentration of intratumoral TGF-β, IL-10, and IL-12 compared to patients without cellular autoimmune responses (Fig. 4a; n = 29). We observed, however, significantly increased levels of intratumoral IFN-α in patients with auto-reactive BMTCs (Fig. 4a; P = 0.0002). All patients without auto-reactive TCs showed IFN-α concentrations below the detection rate of 10 pg/ml, whereas in patients with auto-reactive TCs concentrations above 10 pg/ml have been detected in 63% (Fisher’s exact test; P = 0.0005).
In patients with auto-reactive antibodies, no significant differences were found in the concentration of intratumoral TGF-β and IL-10 compared to patients without humoral autoimmune reactivity (Fig. 4b; n = 19) nor did we detect significant differences regarding intratumoral IFN-α. We observed, however, significantly increased levels of intratumoral IL-12 in patients with humoral autoimmune responses (Fig. 4b; P = 0.04). All patients without auto-reactive antibodies showed IL-12 concentrations below the MDD of 0.5 pg/ml, whereas in patients with auto-reactive antibodies, we detected concentrations above 0.5 pg/ml in 54% (Fisher’s exact test: P = 0.04).
Discussion
Tumor-associated autoimmune disorders represent a relevant issue in clinical practice. According to several studies, a link between autoimmune disease and cancer has to be assumed, showing increased incidence of malignant disease on the basis of autoimmune disorders as well as autoimmune disease following neoplasia [22]. Interestingly, in the literature, there are numerous reports on the clinical course of paraneoplastic syndromes paralleling that of associated solid and hematologic tumor entities with a marked regression of paraneoplastic manifestations usually resulting from medical or surgical tumor treatment [23–25]. During the last years, it has been demonstrated that malignant tumors can be recognized by the host`s immune system revealing an innate, specific recognition of tumor antigens [8–12]. This raises the question whether specific anti-tumor immunity reflects autoimmune responses or whether autoimmune disorders follow anti-tumor immune responses as a by-standing effect. We here demonstrate for the first time a significant correlation between anti-tumor BMTC immunity and cellular autoimmune responses in primary breast cancer patients. Strikingly, spontaneous autoimmune responses were determined by specific pathobiological features of the primary tumor, namely good differentiation and hormone receptor expression, as well as by distinct intratumoral concentrations of IFN-α and IL-12. These observations suggest that in breast cancer patients systemic cellular autoimmune responses may be triggered by the tumor microenvironment, as the induction of these autoimmune responses is determined by conditions similar to those that determine the spontaneous induction of tumor-specific T-cell responses in breast cancer patients [15].
In malignant cells, a variety of proteins are changed structurally. They are presented as peptides via MHC on the tumor cell [26]. Those tumor-associated antigens are potential targets of specific, autologous T-cells. Tumor-associated antigens, however, often belong to a group of commonly shared self-antigens [27]. For instance, tumor-associated antigen MUC-1 acts as a potential target for specific, autologous TCs [28]. It can also be detected, however, on the luminal–apical pole of non-malignant cells in glandular epithelial tissue as for example sebaceous glands or eccrine and apocrine glands of the skin [29, 30]. Various other potential antigens as for example collagens, type III intermediary filament vimentin and laminin or cytokeratins are expressed in both breast cancer and non-malignant tissue such as the skin [31–34]. Even Her-2/neu antigen, as a potent inducer of T-cell immunity, is a self-protein expressed in a variety of tissues of epithelial origin and also detectable in benign breast tissue [35].
In the present study, we detected a slightly higher prevalence of tumor-reactive BMTCs (30%) in comparison with auto-reactive TCs (22%) in primary breast cancer patients, assessing a broad repertoire of tumor- and auto-reactive CD8+ and CD4+ T-cells in tests performed with autologous tumor and skin lysates [10]. This is in striking accordance with previous results, where we detected reactivity against HLA-A*0201-restricted tumor-associated antigens in 33% of all patients, while reactivity against normal breast tissue-associated antigens was encountered in 24% of primary breast cancer patients [14]. Therefore, breast carcinoma cells seem to be more immunogenic than non-malignant cells from epithelial or stromal cell tissue. This might be due to extensive release of matrix-degrading enzymes, heat shock proteins, or danger signals that may activate antigen-presenting cells within neoplastic tissue. Furthermore, due to a high turnover rate of apoptotic tumor cells, there might be a higher amount of tumor cell-derived antigens triggering respective TC responses [14].
We here, furthermore, detected a significantly negative correlation between anti-tumor BMTC immunity and humoral autoimmune responses. We observed a dichotomy in immune responses, showing two groups of patients with either cellular tumor immunity in combination with cellular autoimmune reactivity or patients with humoral autoimmune responses in the absence of tumor-reactive BMTCs. This is in striking accordance with earlier findings [15], where we also detected a dichotomy of cellular and humoral immune responses against tumor antigens. In this previous work, a majority of patients without tumor-reactive TCs showed tumor antigen-specific antibodies, whereas only few patients with tumor-specific type 1 BMTC response produced TA-specific antibodies [15].
Parallel to our previous work [15], where we detected tumor-specific BMTCs predominantly in patients with well-differentiated, hormone receptor-positive carcinomas, we now observe an identical impact of pathobiological primary tumor features. Again, especially patients with low graded, hormone receptor-positive carcinomas showed cellular autoimmune responses in the form of auto-reactive BMTCs. Interestingly, tumor-associated MUC-1 as a potential target for specific, autologous TCs, which is also expressed on the luminal–apical pole of non-malignant cells in glandular epithelial tissue, is mainly found in carcinomas with a lower grade and a higher estrogen receptor (ER)-positive phenotype [36].
Furthermore, we recently detected distinguished intratumoral cytokine profiles in breast cancer tissue, showing a significantly increased content of intratumoral IFN-α in well differentiated, low-graded breast cancer in strong correlation to the presence of tumor-reactive BMTCs [15]. Strikingly, in the present study, spontaneous auto-reactive TC immunity was also determined by elevated levels of intratumoral IFN-α, which is a major inducer of DC activation, DC homing to lymphoid organs, and efficient priming of type 1 CD8+ and CD4+ TC responses [37]. The ability of IFN-α to induce autoimmunity and exacerbate Th1 diseases is well known [38]. Accordingly, several case reports have emerged describing autoimmune conditions that have developed during IFN-α therapy [39]. IFN-α can synergistically amplify T-cell autoreactivity by directly promoting T-cell activation and keeping activated T-cells alive [40]. In parallel, IFN-α suppresses the generation of CD4(+) FoxP3(HI)-activated regulatory TCs [41], thus potentially pushing immune homeostasis toward autoimmunity.
Eventually, we detected humoral autoimmune reactivity especially in patients with increased levels of IL-12 in cancer tissue. IL-12 is binding directly to B cells, stimulating IL-2-dependent differentiation toward plasma cells and thereby the secretion of IgM [42]. IL-12 is considered the most potent enhancer of IL-2-dependent immunoglobulin secretion by B cells. This suggests an essential role of IL-12 in the regulation of humoral immune responses with regard to their extent [43, 44]. For instance, patients with clinically symptomatic lupus erythematosus have recently been shown to present increased levels of IL-12 or IL-12-induced IL-18 in the peripheral blood. In parallel, the presence of anti-nuclear antibodies is correlated with increased levels of serum IL-12/IL-18 [45–47]. Interestingly, IFN-α can inhibit the production of active IL-12, whereas the neutralization of IFN-α has been demonstrated to enhance IL-12 production [48], thus possibly explaining the observed dichotomy of humoral and cellular autoimmune responses.
In conclusion, we here report a significant correlation between anti-tumor immunity and autoimmune phenomena in primary breast cancer patients. We show specific pathobiological features of the primary tumor as well as distinct intratumoral concentrations of IFN-α and IL-12 that turned out to have significant impact on the induction of cellular and humoral autoimmune responses. These findings suggest an essential role of the tumor microenvironment in the induction of systemic autoimmune responses and might imply the primary tumor tissue to be the integral site of paraneoplastic induction of autoimmunity.
Acknowledgments
Conflict of interest
None declared.
Abbreviations
- ANA
Anti-nuclear antibody
- ANCA
Anti-neutrophil cytoplasmic antibody
- BM/BMTC
Bone marrow/bone marrow T-cell
- CD
Cluster of differentiation
- DC
Dendritic cell
- EliSpot
Enzyme-linked immunosorbent spot
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- IFN
Interferon
- IL
Interleukin
- PBMC
Peripheral blood mononuclear cell
- RhF
Rheumatoid factor
- TA
Tumor antigen
- TC
T-cell
- TgAb
Anti-thyreoglobulin antibody
- TGF
Transforming growth factor
- TRAb
Thyroid-stimulating hormone receptor antibody
Footnotes
C. Domschke and F. Schuetz contributed equally.
References
- 1.Tishler M, Shoenfeld Y. Paraneoplastic syndromes. In: Shoenfeld Y, Gershwin ME, editors. Cancer and autoimmunity. Amsterdam: Elsevier; 2000. pp. 121–133. [Google Scholar]
- 2.Conrad K. Autoantibodies in cancer patients and in persons with a higher risk of cancer development. In: Shoenfeld Y, Gershwin ME, editors. Cancer and autoimmunity. Amsterdam: Elsevier; 2000. pp. 159–174. [Google Scholar]
- 3.Gatti G, Simsek S, Kurne A, Zurrida S, Naninato P, Veronesi P, Frasson A, Millen E, Rososchansky J, Luini A. Paraneoplastic neurological disorders in breast cancer. Breast. 2003;12:203–207. doi: 10.1016/S0960-9776(03)00011-0. [DOI] [PubMed] [Google Scholar]
- 4.Tomer Y, Sherer Y, Shoenfeld Y. Autoantibodies, autoimmunity and cancer (review) Oncol Rep. 1998;5:753–761. doi: 10.3892/or.5.3.753. [DOI] [PubMed] [Google Scholar]
- 5.Turken O, Narin Y, Demirbas S, Onde ME, Sayan O, Kandemir EG, Yaylaci M, Ozturk A. Breast cancer in association with thyroid disorders. Breast Cancer Res. 2003;5:R110–R113. doi: 10.1186/bcr609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zantos D, Zhang Y, Felson D. The overall and temporal association of cancer with polymyositis and dermatomyositis. J Rheumatol. 1994;21:1855–1859. [PubMed] [Google Scholar]
- 7.Jiskra J, Barkmanova J, Limanova Z, Lánská V, Smutek D, Potlukova E, Antosova M. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol Rep. 2007;18:1603–1611. [PubMed] [Google Scholar]
- 8.Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi C, Witzens M, Bucur M, Feuerer M, Sommerfeldt N, Trojan A, Ho A, Schirrmacher V, Goldschmidt H, Beckhove P. Enrichment of functional CD8 memory T cells specific for MUC1 in bone marrow of patients with multiple myeloma. Blood. 2005;105:2132–2134. doi: 10.1182/blood-2004-01-0366. [DOI] [PubMed] [Google Scholar]
- 10.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 11.Nagorsen D, Scheibenbogen C, Marincola FM, Letsch A, Keilholz U. Natural T cell immunity against cancer. Clin Cancer Res. 2003;9:4296–4303. [PubMed] [Google Scholar]
- 12.Schmitz-Winnenthal FH, Volk C, Z’graggen K, Galindo L, Nummer D, Ziouta Y, Bucur M, Weitz J, Schirrmacher V, Büchler MW, Beckhove P. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 13.Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, Engels K, Karn T, Kaufmann M. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11:R15. doi: 10.1186/bcr2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommerfeldt N, Schütz F, Sohn C, Förster J, Schirrmacher V, Beckhove P. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res. 2006;66:8258–8265. doi: 10.1158/0008-5472.CAN-05-4201. [DOI] [PubMed] [Google Scholar]
- 15.Domschke C, Schuetz F, Ge Y, Seibel T, Falk C, Brors B, Vlodavsky I, Sommerfeldt N, Sinn HP, Kühnle MC, Schneeweiss A, Scharf A, Sohn C, Schirrmacher V, Moldenhauer G, Momburg F, Beckhove P. Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Res. 2009;69:8420–8428. doi: 10.1158/0008-5472.CAN-09-1627. [DOI] [PubMed] [Google Scholar]
- 16.Domschke C, Neubrech F, Dick M, Rom J, Beckhove P, Sohn C, Schuetz F, Scharf A. Intraoperative bone marrow puncture in breast cancer patients: Prospective assessment of adverse side-effects. Breast. 2010 doi: 10.1016/j.breast.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Bai L, Feuerer M, Beckhove P, Umansky V, Schirrmacher V. Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. Int J Oncol. 2002;20:247–253. [PubMed] [Google Scholar]
- 18.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Müller-Berghaus J, Ehlert K, Ugurel S, Umansky V, Bucur M, Schirrmacher V, Beckhove P, Schadendorf D. Melanoma-reactive T cells in the bone marrow of melanoma patients: association with disease stage and disease duration. Cancer Res. 2006;66:5997–6001. doi: 10.1158/0008-5472.CAN-04-0484. [DOI] [PubMed] [Google Scholar]
- 20.Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008;20:663–668. doi: 10.1016/j.coi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis. 2001;60:433–441. doi: 10.1136/ard.60.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racanelli V, Prete M, Minoia C, Favoino E, Perosa F. Rheumatic disorders as paraneoplastic syndromes. Autoimmun Rev. 2008;7:352–358. doi: 10.1016/j.autrev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev. 2009;8:410–414. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sands J, Tuscano JM. Geoepidemiology and autoimmune manifestations of lymphoproliferative disorders. Autoimmun Rev. 2010;9:A335–A341. doi: 10.1016/j.autrev.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 27.Wang RF. Human tumor antigens: implications for cancer vaccine development. J Mol Med. 1999;77:640–655. doi: 10.1007/s001099900042. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee P, Ginardi AR, Tinder TL, Sterner CJ, Gendler SJ. MUC1-specific cytotoxic T lymphocytes eradicate tumors when adoptively transferred in vivo. Clin Cancer Res. 2001;7:848s–855s. [PubMed] [Google Scholar]
- 29.Leroy X, Buisine MP, Leteurtre E, Aubert S, Buob D, Porchet N, Copin MC. [MUC1 (EMA): a key molecule of carcinogenesis?] Ann Pathol. 2006;26:257–266. doi: 10.1016/s0242-6498(06)70718-0. [DOI] [PubMed] [Google Scholar]
- 30.Yoshii N, Kitajima S, Yonezawa S, Matsukita S, Setoyama M, Kanzaki T. Expression of mucin core proteins in extramammary Paget’s disease. Pathol Int. 2002;52:390–399. doi: 10.1046/j.1440-1827.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Pinilla SM, Sarrió D, Honrado E, Moreno-Bueno G, Hardisson D, Calero F, Benítez J, Palacios J. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007;60:1006–1012. doi: 10.1136/jcp.2006.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kauppila S, Stenbäck F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Eerola H, Heinonen M, Heikkilä P, Kilpivaara O, Tamminen A, Aittomäki K, Blomqvist C, Ristimäki A, Nevanlinna H. Basal cytokeratins in breast tumours among BRCA1, BRCA2 and mutation-negative breast cancer families. Breast Cancer Res. 2008;10:R17. doi: 10.1186/bcr1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehr HA, Folpe A, Yaziji H, Kommoss F, Gown AM. Cytokeratin 8 immunostaining pattern and E-cadherin expression distinguish lobular from ductal breast carcinoma. Am J Clin Pathol. 2000;114:190–196. doi: 10.1309/CPUX-KWEH-7B26-YE19. [DOI] [PubMed] [Google Scholar]
- 35.Stark A, Hulka BS, Joens S, Novotny D, Thor AD, Wold LE, Schell MJ, Melton LJ, 3rd, Liu ET, Conway K. HER-2/neu amplification in benign breast disease and the risk of subsequent breast cancer. J Clin Oncol. 2000;18:267–274. doi: 10.1200/JCO.2000.18.2.267. [DOI] [PubMed] [Google Scholar]
- 36.Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 37.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 38.Monteleone G, Pender SL, Wathen NC, MacDonald TT. Interferon-alpha drives T cell-mediated immunopathology in the intestine. Eur J Immunol. 2001;31:2247–2255. doi: 10.1002/1521-4141(200108)31:8<2247::AID-IMMU2247>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Selmi C, Lleo A, Zuin M, Podda M, Rossaro L, Gershwin ME. Interferon alpha and its contribution to autoimmunity. Curr Opin Investig Drugs. 2006;7:451–456. [PubMed] [Google Scholar]
- 40.Conrad B. Potential mechanisms of interferon-alpha induced autoimmunity. Autoimmunity. 2003;36:519–523. doi: 10.1080/08916930310001602137. [DOI] [PubMed] [Google Scholar]
- 41.Golding A, Rosen A, Petri M, Akhter E, Andrade F. Interferon-alpha regulates the dynamic balance between human activated regulatory and effector T cells: implications for antiviral and autoimmune responses. Immunology. 2010;131:107–117. doi: 10.1111/j.1365-2567.2010.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel LA, Lester TL, Van Cleave VH, Metzger DW. Inhibition of murine B1 lymphocytes by interleukin-12. Eur J Immunol. 1996;26:219–223. doi: 10.1002/eji.1830260134. [DOI] [PubMed] [Google Scholar]
- 43.Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 44.Jelinek DF, Braaten JK. Role of IL-12 in human B lymphocyte proliferation and differentiation. J Immunol. 1995;154:1606–1613. [PubMed] [Google Scholar]
- 45.Mosaad YM, Metwally SS, Auf FA, AbdEL-Samee ER, el-Deek B, Limon NI, el-Chennawi FA. Proinflammatory cytokines (IL-12 and IL-18) in immune rheumatic diseases: relation with disease activity and autoantibodies production. Egypt J Immunol. 2003;10:19–26. [PubMed] [Google Scholar]
- 46.Robak E, Robak T, Wozniacka A, Zak-Prelich M, Sysa-Jedrzejowska A, Stepien H. Proinflammatory interferon-gamma–inducing monokines (interleukin-12, interleukin-18, interleukin-15)–serum profile in patients with systemic lupus erythematosus. Eur Cytokine Netw. 2002;13:364–368. [PubMed] [Google Scholar]
- 47.Maczynska I, Millo B, Ratajczak-Stefańska V, Maleszka R, Szych Z, Kurpisz M, Giedrys-Kalemba S. Proinflammatory cytokine (IL-1beta, IL-6, IL-12, IL-18 and TNF-alpha) levels in sera of patients with subacute cutaneous lupus erythematosus (SCLE) Immunol Lett. 2006;102:79–82. doi: 10.1016/j.imlet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]


