Fig. 5.
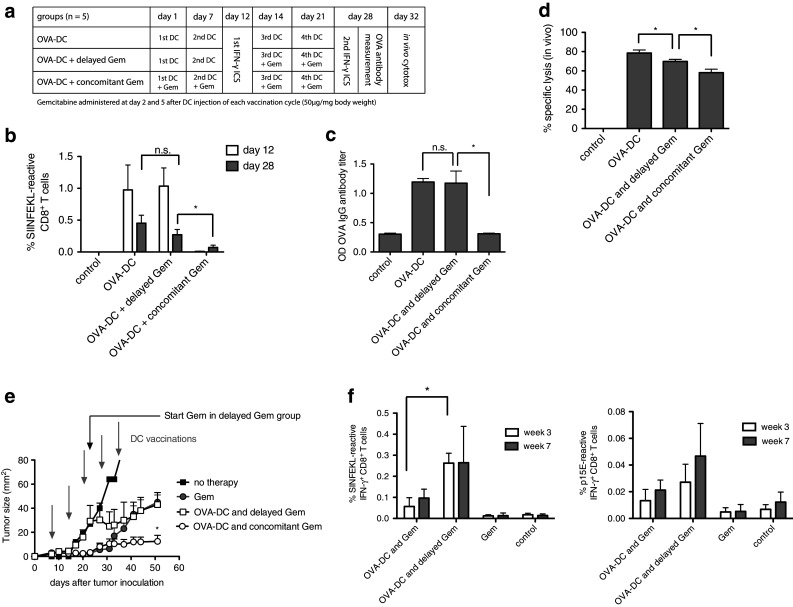
Delayed gemcitabine administration avoids chemotherapy-induced B- and T-cell suppression. a Treatment and immune monitoring scheme. OVA-DC-vaccinated mice were divided into three groups: OVA-DC with concomitant gemcitabine treatment (OVA-DC and concomitant Gem), delayed start of chemotherapy (OVA-DC and delayed Gem) and no chemotherapy (OVA-DC). b OVA-specific CD8+ T-cell responses were measured after the second DC vaccination (before the start of gemcitabine in “OVA-DC and delayed Gem” group) and at the end of the experimental protocol after four vaccinations by IFN-γ ICS assay. c Anti-OVA IgG antibody titers were assessed after four cycles of DC treatment. d In vivo OVA-specific CTL-mediated cytotoxicity was examined by measurement of target-cell lysis 20 h after adoptive transfer of CFSE-labeled, SIINFEKL-loaded splenocytes. e Mice were injected s.c. with 106 PancOVA cells and treated with Gem alone or OVA-DC ± Gem. Gray arrows indicate DC vaccinations; a black arrow indicates the start of gemcitabine therapy in the “OVA-DC and delayed Gem” group. f OVA- and p15E-specific CD8+ T-cell responses were determined before the start (white bars) and after the start of chemotherapy (gray bars) in the “OVA-DC and delayed Gem” group. Figure 4e, f represents one of two independent experiments (n = 7 per group)
