Abstract
Uterine leiomyosarcoma comprises <1 % of uterine malignancies and is known for its clinically aggressive course. Extrapelvic recurrences are common and often lethal. No adjuvant therapies have been shown to significantly improve overall survival, highlighting the need for new and novel therapies. Our objective was to determine whether GD2-specific immunocytokine therapy may be explored for the treatment for uterine leiomyosarcoma. To do so, frozen tissue sections were obtained from the Gynecologic Oncology Group tumor bank and evaluated by immunohistochemistry (IHC) for GD2 expression using both the parent mouse monoclonal antibody 14G2A and immunocytokine 14.18-IL2 generated from the 14G2A sequence. Immunoreactivity was detected by avidin–biotin complex with DAB substrate. Specimens were reviewed by a pathologist with light microscopy and classified as negative, 1+, 2+ or 3+, compared to human melanoma cells as positive control and tissue incubated in the absence of primary antibody as negative control. GD2 was diffusely present in all evaluable samples. 10 tumors (67 %) demonstrated 3+ IHC intensity for GD2, two tumors (13 %) demonstrated 2+ intensity, and 3 (20 %) tumors demonstrated 1+ intensity. Eleven cases had sufficient tissue to assess 14.18-IL2 binding. All 11 cases bound 14.18-IL2 in a pattern identical to the parent antibody. Uterine leiomyosarcoma diffusely express GD2 and bind the therapeutic immunocytokine 14.18-IL2. This warrants further exploration to determine whether immunocytokine therapy may have a clinical role in the management of these aggressive tumors.
Keywords: 14.18, Uterine leiomyosarcoma, Immunotherapy, GD2, Sarcoma
Introduction
Uterine leiomyosarcoma is a rare, aggressive mesenchymal tumor comprising approximately 1 % of uterine cancers [1]. These tumors arise de novo from uterine smooth muscle and are microscopically characterized by hypercellularity, abundant mitoses, marked atypia, and large areas of necrosis. Surgical intervention remains gold standard for primary treatment. However, despite surgical resection, 66 % of women with stage I/II disease will succumb to their disease within 5 years. Recurrence is common, occurring in 50 % of women diagnosed with well-staged early-stage disease, and in over 80 % of women with advanced stage disease, despite aggressive resection at the time of primary surgery. Of those patients with recurrence, 40 % have extrapelvic disease. These patients are rarely cured, drawing attention to the need for effective systemic adjuvant therapy capable of clearing sub-clinical disease. Despite extensive study of adjuvant hormones, radiotherapy, and multiple chemotherapeutic regimens, no adjuvant therapies have been shown to significantly impact overall survival, and no consensus regarding preferred adjuvant modalities has been reached [2].
Globally, cancer research paradigms are shifting away from empiric cytotoxic pharmacotherapeutics toward more targeted therapies. One such specific target has been the disialoganglioside GD2. Gangliosides are acidic class of glycolipids expressed on cell membranes. Typically, GD2 is expressed in cerebellar neurons, peripheral pain fibers, and melanocytes. GD-2 has also been found to be over-expressed in tumors of neuroectodermal origin, including neuroblastoma, melanoma, small cell lung cancers, and soft-tissue sarcomas [3–5]. Specifically, in a series of 39 soft-tissue sarcomas including liposarcomas, fibrosarcomas, malignant fibrous histiocytomas, spindle cell sarcomas, and 10 leiomyosarcomas, GD2 was found to display diffuse anti-GD2 staining in nearly 95 % of specimen as evaluated by immunohistochemistry [4]. However, the majority of these were nongynecologic specimen. Because GD-2 is so selectively over-expressed in these tumors, it has been a promising target for antibody-directed therapy.
Initially, anti-GD2 antibody, chimeric14.18 (ch14.18), was fused with cytokines including GM-CSF and IL-2 to produce molecules called immunocytokines (ICs). These early ICs were then used successfully as targeted therapy in animal models [6, 7]. Subsequently, humanized 14.18 has been complexed with IL-2 (14.18-IL2) and tested in a phase I and II clinical trials in children with refractory or recurrent neuroblastoma and adults with melanoma [8–10]. These studies have established hu14-18-IL2s pharmacokinetics, administration regimen, and maximal tolerated doses and have demonstrated an acceptable toxicity profile in both adult and pediatric populations [9, 11–13]. GD2 research has been most significantly impacted by a recent phase III trial, which showed that children with high-risk neuroblastoma and minimal residual disease had significantly improved outcomes when treated adjuvantly with ch14.18, GM-CSF, and interleukin-2 [14].
The success of recent clinical trials targeting GD2, the presence of GD2 in other soft-tissue sarcomas, and the paucity of effective systemic adjuvant treatments for uterine LMS led us to evaluate GD-2 expression in uterine leiomyosarcoma, to determine whether GD2-directed therapy may be worthwhile for investigation as a treatment for this highly aggressive disease.
Methods and materials
After institutional IRB approval was obtained, all available cases of uterine LMS with frozen tissue available were obtained from the Gynecologic Oncology Group (GOG) tumor bank. Frozen sections were mounted on charged slides, fixed in cold acetone, and blocked for 30 min in normal serum. Sections were incubated with the anti-GD2 primary mouse monoclonal antibody, 14G2A (Dr. Paul Sondel, University of Wisconsin Hospitals and Clinics, Madison, WI, USA) at 30 μg/ml for 4 h at room temperature. Sections were washed and incubated with biotinylated secondary antibody for 30 min at room temperature. Immunoreactivity was detected by avidin–biotin complex (ABC) with DAB substrate (Vector Labs, Burlingame, CA, USA). Positive control sections of human melanoma and negative control sections from each tumor incubated in the absence of primary antibody were evaluated. Nonspecific isotype IgG controls were performed in a subset of cases. Sections were evaluated by a gynecologic pathologist, using light microscopy to determine the presence and pattern of specific staining. The specimens were qualitatively scored as negative, 1+, 2+ or 3+. Simple descriptive statistics were performed.
Those samples with sufficient tissue remaining were incubated with the therapeutic immunocytokine 14.18-IL2 (Merck-Serono, Darmstadt, Germany) for 4 h at room temperature. Sections were then washed and incubated with biotinylated mouse anti-human IL2 IgG. Once again, immunoreactivity was determined by ABC/DAB, and sections were reviewed and evaluated by light microscopy as described above.
Results
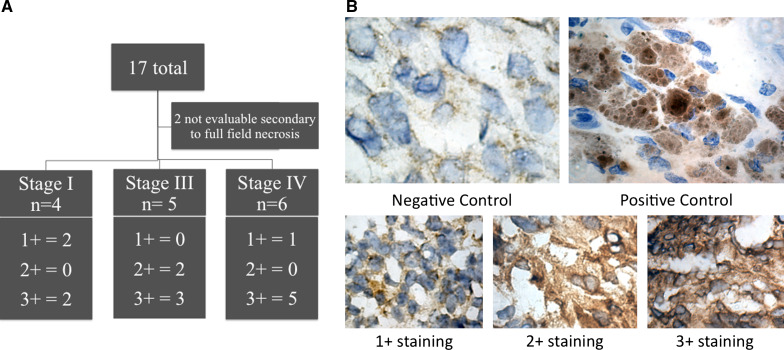
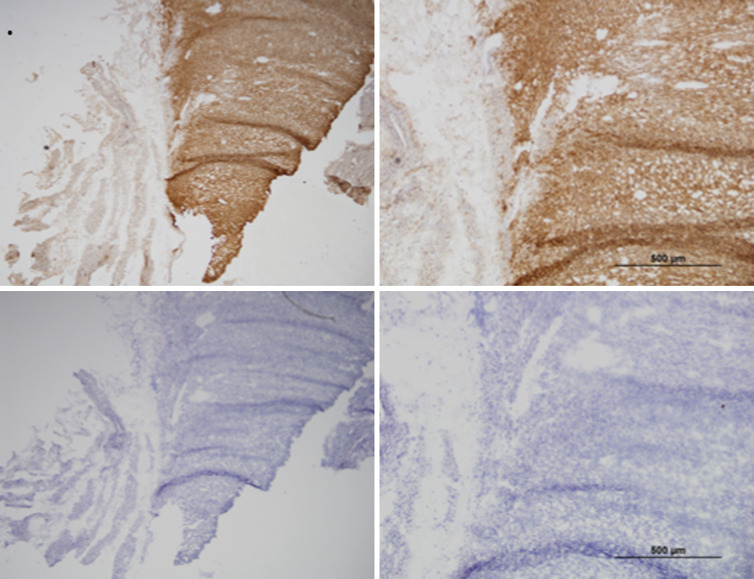
Tumors analyzed are outlined in Fig. 1. Two specimens from advanced stage cancers contained nearly full-field necrosis resulting in strong nonspecific staining and were therefore not interpretable, leaving 15 evaluable specimens. Both positive and negative controls were evaluated and found to be appropriate for comparison. Of the 15 evaluable tumors, four were from patients with stage I disease, 5 were from patients with stage III disease, and 6 were from patients with stage IV disease (Fig. 1a). Of evaluable specimens, 10 tumors (67 %) demonstrated 3+ immunohistochemical intensity for GD2 (stage I = 2, III = 3, IV = 5), two tumors (13 %) demonstrated 2+ intensity (stage III = 2), and 3 tumors (20 %) demonstrated 1+ intensity (stage I = 2, IV = 1). Representative photomicrographs can be seen in Fig. 1b. No tumors were characterized as GD2-negative. Overall, GD2 staining was characterized as diffusely positive, without patchy or isolated GD2 expression (Fig. 2). Additionally, each of 11 samples with adequate tissue for immunohistochemistry for 14.18-IL2 was noted to bind the immunocytokine in a pattern identical to the parent mouse monoclonal antibody 14G2A. While normal myometrial stroma was not present in the majority of specimens, owning to the large size of gross specimen, those with residual myometrium revealed negative GD2 staining.
Fig. 1.
a Table summarizing stage and immunohistochemistry results. b Photomicrographs taken at ×100 magnification of uterine leiomyosarcoma incubated without secondary antibody serving as negative control, anti-GD2-stained malignant melanoma as positive control, and representative uterine leiomyosarcoma sections showing 1+ (case 1), 2+ (case 8), and 3+ (case 3) anti-GD2 staining with the mouse monoclonal antibody 14G2A
Fig. 2.
Serial sections of LMS incubated without primary antibody as a negative control at 5× and 10×, respectively (first row), and with 14G2A mouse monoclonal antibody (second row)
Discussion
Uterine LMS, though rare, is a devastating gynecologic malignancy. Despite excision, recurrence rates exceed 50 % in early-stage disease and 80 % in late-stage disease. While postoperative adjuvant radiation and chemotherapy has been studied extensively, there are no adjuvant treatments effective in preventing distant recurrence. Likewise, no prospective trials have found therapies capable of improving survival in patients with recurrent or persistent disease.
Radiotherapy is of limited utility in the treatment for uterine LMS. While it has been shown to improve local control, it has shown no improvement in overall survival in patients with early-stage sarcoma [1, 15–17]. A randomized controlled study was performed by the EORGTC randomized patients with early-stage uterine sarcomas to adjuvant whole pelvic radiation and found improvement in local control. However, subgroup analysis of patients with LMS associated radiation therapy with an increased rate of recurrence as compared to those in the observation group [1].
Uterine LMS is also quite chemoresistant: Single-agent chemotherapeutics including ifosfamide, doxorubicin, dacarbazapine, cisplatin, and paclitaxel have been shown to have relatively poor activity against LMS, none exceeding response rates of 25 % [18–21]. Doxorubicin and ifosfamide used in tandem have been shown to have partial response rates, ranging from 30 to 55 % [22]. Perhaps most promising in the cytotoxic treatment for uterine LMS is the fixed-dose regimen of docetaxel and gemcitabine, which has been shown to improve progression-free survival patients in patients with advanced uterine LMS [23, 24]. In short, while it is reasonable to offer systemic chemotherapy either as adjuvant treatment to affected patients, no therapies have been shown to impact overall survival, emphasizing the need for developing novel modalities for the treatment for this disease.
Our study shows that uterine leiomyosarcomas diffusely express GD2 and subsequently bind the anti-GD2 therapeutic immunocytokine 14.18-IL2. There are certain limitations to this small descriptive study. Though we were able to obtain all specimen from a national tissue repository, uterine LMS is rare. Intraoperatively, lesions are frequently mistaken for benign leiomyoma, so frozen specimens are rarely sent for evaluation, and the diagnosis of uterine LMS is most frequently made on formalin-fixed, paraffin-embedded tissue. However, GD2 is degraded by formalin, and frozen sections performed on advanced disease are more likely to have necrosis limiting IHC evaluation. Necrotic areas are prone to extensive nonspecific staining, which further limits accurate evaluation. Therefore, our numbers are limited both by rarity and by difficulties with specimen processing. Additionally, there are no animal orthotopic models for uterine LMS, and attempts to produce uterine LMS cell lines have been futile, further limiting the potential for additional preclinical study of GD2-directed therapy in this disease. Therefore, we must look to anti-GD2 therapies that have been studied in other malignancies for assistance with applying treatment to patients with uterine LMS.
To date, several GD-2 targeted therapies have been studied in the other solid tumors, namely melanoma and neuroblastoma. Monoclonal antibodies specific to GD2 carrying cationic liposomes containing ifosfamide have studied in vitro in the treatment for both tumor types [25]. Additionally, radiotherapy with a radiolabeled GD2-specific mAb has also been shown to be feasible and is being studied in a phase II trial as one method to protect patients from dose-dependant radiation toxicity. Active immunizations against GD2 have also been pursued, however, because carbohydrates are T cell-independent antigen, only weak immune responses have been elicited. To overcome this, gangliosides have been coupled to carrier molecules in effort to induce a more substantial response [26].
A recent phase II study sponsored by the Children’s Oncology Group evaluated the use of 14.18-IL2 in children with recurrent/refractory neuroblastoma. Intravenous Hu14.18-IL2 (12 mg/m2/daily) was administered every 4 weeks for 3 days in patients with biochemically measurable recurrent/refractory disease. Of 23 patients evaluable only by [123I]metaiodobenzylguanidine (MIBG) scintigraphy and/or bone marrow (BM) histology, five patients (21.7 %) demonstrated complete response of durable duration (9, 13, 20, 30, and 35 months) [27]. Another phase III trial evaluated 226 patients with high-risk neuroblastoma who had a response to induction chemotherapy and stem cell transplantation. Patients were randomized to either six cycles of isotretinoin or six cycles of isotretinoin + immunotherapy with ch14.18 in combination with alternating GM-CSF and interleukin-2. The trial was terminated early, following 61 % of the number of expected events observed secondary to treatment efficacy. Immunotherapy was found to be superior when compared to standard therapy in terms of recurrence free (66 ± 5 % vs. 46 ± 5 % at 2 years, P = 0.01) and overall survival (86 ± 4 % vs. 75 ± 5 % at 2 years, P = 0.02). However, a total of 52 % of patients undergoing immunotherapy had grade 3 or higher pain, 23 % had capillary leak syndrome and 25 % had hypersensitivity reactions [14]. This is one of the first trials to demonstrate treatment efficacy in neuroblastoma and represents a major advancement in the immunotherapy of cancer.
There are many similarities between these neuroblastoma patients and both early-stage and optimally debulked LMS patients. Anti-GD2 therapy provides optimal benefit (in both in vivo animal models and human clinical trials) in the context of minimal residual, sub-clinical disease. The high rates of recurrence in uterine LMS demonstrate that large numbers of women have residual sub-clinical disease, underscoring the need for effective adjuvant therapy. The high expression of GD2 in uterine LMS and its restricted distribution in normal tissues make anti-GD2 therapies potentially suitable for immunotherapy. Because LMS express GD2 and diffusely binds the immunocytokine, 14.18-IL2, further exploration is warranted to determine whether this novel immunocytokine or other GD2-directed therapies could be used adjuvantly in the treatment for uterine LMS.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44(6):808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Sleijfer S, Seynaeve C, Verweij J. Gynaecological sarcomas. Curr Opin Oncol. 2007;19(5):492–496. doi: 10.1097/CCO.0b013e3282748eaa. [DOI] [PubMed] [Google Scholar]
- 3.Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45(6):2642–2649. [PubMed] [Google Scholar]
- 4.Chang HR, Cordon-Cardo C, Houghton AN, Cheung NK, Brennan MF. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer. 1992;70(3):633–638. doi: 10.1002/1097-0142(19920801)70:3<633::AID-CNCR2820700315>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Heiner JP, Miraldi F, Kallick S, et al. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res. 1987;47(20):5377–5381. [PubMed] [Google Scholar]
- 6.Batova A, Kamps A, Gillies SD, Reisfeld RA, Yu AL. The Ch14.18-GM-CSF fusion protein is effective at mediating antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro. Clin Cancer Res. 1999;5(12):4259–4263. [PubMed] [Google Scholar]
- 7.Hank JA, Surfus JE, Gan J, et al. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14.18-IL2) Clin Cancer Res. 1996;2(12):1951–1959. [PubMed] [Google Scholar]
- 8.Frost JD, Hank JA, Reaman GH, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children’s Cancer Group. Cancer. 1997;80(2):317–333. doi: 10.1002/(SICI)1097-0142(19970715)80:2<317::AID-CNCR21>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.King DM, Albertini MR, Schalch H, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22(22):4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleh MN, Khazaeli MB, Wheeler RH, et al. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum Antibodies Hybridomas. 1992;3(1):19–24. [PubMed] [Google Scholar]
- 11.Hank JA, Surfus JE, Gan J, Ostendorf A, Gillies SD, Sondel PM. Determination of peak serum levels and immune response to the humanized anti-ganglioside antibody-interleukin-2 immunocytokine. Methods Mol Med. 2003;85:123–131. doi: 10.1385/1-59259-380-1:123. [DOI] [PubMed] [Google Scholar]
- 12.Kendra K, Gan J, Ricci M, et al. Pharmacokinetics and stability of the ch14.18-interleukin-2 fusion protein in mice. Cancer Immunol Immunother. 1999;48(5):219–229. doi: 10.1007/s002620050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin Cancer Res. 2006;12(6):1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadducci A, Cosio S, Romanini A, Genazzani AR. The management of patients with uterine sarcoma: a debated clinical challenge. Crit Rev Oncol Hematol. 2008;65(2):129–142. doi: 10.1016/j.critrevonc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Koivisto-Korander R, Leminen A, Heikinheimo O. Mifepristone as treatment of recurrent progesterone receptor-positive uterine leiomyosarcoma. Obstet Gynecol. 2007;109(2 Pt2):512–514. doi: 10.1097/01.AOG.0000223228.23289.0f. [DOI] [PubMed] [Google Scholar]
- 17.Verleye L, Ottevanger PB, van der Graaf W, Reed NS, Vergote I. EORTC-GCG process quality indicators for ovarian cancer surgery. Eur J Cancer. 2009;45(4):517–526. doi: 10.1016/j.ejca.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Muss HB, Bundy B, DiSaia PJ, et al. Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group) Cancer. 1985;55(8):1648–1653. doi: 10.1002/1097-0142(19850415)55:8<1648::AID-CNCR2820550806>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Omura GA, Major FJ, Blessing JA, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer. 1983;52(4):626–632. doi: 10.1002/1097-0142(19830815)52:4<626::AID-CNCR2820520409>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Sutton G, Blessing J, Hanjani P, Kramer P. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;96(3):749–752. doi: 10.1016/j.ygyno.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Thigpen T, Blessing JA, Yordan E, Valea F, Vaccarello L. Phase II trial of etoposide in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;63(1):120–122. doi: 10.1006/gyno.1996.0289. [DOI] [PubMed] [Google Scholar]
- 22.Leyvraz S, Zweifel M, Jundt G, et al. Long-term results of a multicenter SAKK trial on high-dose ifosfamide and doxorubicin in advanced or metastatic gynecologic sarcomas. Ann Oncol. 2006;17(4):646–651. doi: 10.1093/annonc/mdl020. [DOI] [PubMed] [Google Scholar]
- 23.Hensley ML, Ishill N, Soslow R, et al. Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: results of a prospective study. Gynecol Oncol. 2009;112(3):563–567. doi: 10.1016/j.ygyno.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109(3):329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brignole C, Marimpietri D, Gambini C, Allen TM, Ponzoni M, Pastorino F. Development of Fab’ fragments of anti-GD(2) immunoliposomes entrapping doxorubicin for experimental therapy of human neuroblastoma. Cancer Lett. 2003;197(1–2):199–204. doi: 10.1016/S0304-3835(03)00099-5. [DOI] [PubMed] [Google Scholar]
- 26.Ragupathi G, Livingston PO, Hood C, et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9(14):5214–5220. [PubMed] [Google Scholar]
- 27.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]