Abstract
Targeting interleukin-2 (IL-2) and/or agonist anti-CD40 antibody (Ab) into tumors represents an effective vaccination strategy that avoids systemic toxicity and resolves treated-site tumors. Here, we examined IL-2 and/or anti-CD40 Ab-driven local versus systemic T cell function and the installation of T cell memory. Single tumor studies showed that IL-2 induced a potent CD4+ and CD8+ T cell response that was limited to the draining lymph node and treated-site tumor, and lymph node tumor-specific CD8+ T cells did not upregulate CD44. A two-tumor model showed that while IL-2-treated-site tumors resolved, distal tumors continued to grow, implying limited systemic immunity. In contrast, anti-CD40 Ab treatment with or without IL-2 expanded the systemic T cell response to non-draining lymph nodes, and distal tumors resolved. Tumor-specific T cells in lymph nodes of anti-CD40 Ab ± IL-2-treated mice upregulated CD44, demonstrating activation and transition to effector/memory migratory cells. While CD40-activated CD4+ T cells were not required for eradicating treated-site tumors, they, plus CD8+ T cells, were crucial for removing distal tumors. Rechallenge/depletion experiments showed that the effector/memory phase required the presence of previously CD40/IL-2-activated CD4+ and CD8+ T cells to prevent recurrence. These novel findings show that different T cell effector mechanisms can operate for the eradication of local treated-site tumors versus untreated distal tumors and that signaling through CD40 generates a whole of body, effector/memory CD4+ and CD8+ T cell response that is amplified and prolonged via IL-2. Thus, successful immunotherapy needs to generate collaborating CD4+ and CD8+ T cells for a complete long-term protective cure.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-011-1120-5) contains supplementary material, which is available to authorized users.
Keywords: Tumor immunity, CD4+ T cells, CD8+ T cells, Memory
Introduction
We, and others, have shown that developing solid tumors communicate with draining LNs (dLNs) leading to the generation of CD8+ cytotoxic T cells (CTL) [1–4]. These cells may traffic into the tumor microenvironment where they are ineffective [5, 6], but once activated appropriately can destroy tumors [7, 8]. This may explain why vaccine strategies that target the tumor microenvironment can be effective [9, 10]. In our models of mesothelioma and lung cancer systemic administration via intravenous (i.v.) or intraperitoneal (i.p.), injection of either IL-2 or agonist anti-CD40 Ab was less effective and required higher toxic doses than intratumoral (i.t.) delivery [11]. The intratumoral (i.t.) IL-2 or anti-CD40 Ab monotherapies were effective when faced with small tumor burdens [12, 13], and eradicating larger tumor burdens required local co-administration of IL-2 with anti-CD40 Ab [11]. Importantly, promising effects have been observed after local administration of immune-enhancing agents into mesothelioma tumors via direct injection or via intrapleural gene therapy in clinical trials [14, 15]. Encouragingly, technological advances such as CT-guided injections, gene therapy, monoclonal antibodies, and nanotechnology will further enable tumor targeting [16, 17].
Few studies have systematically examined the impact of intratumoral immunotherapy on local versus global (systemic) antitumor immunity and long-term memory. While agonist anti-CD40 Ab treatment has been shown to bypass the need for CD4+ help in the priming [18, 19] and effector/memory phases [20, 21], in several studies, others have shown that regardless of CD40 activation, CD4+ T cell help for CD8+ T cells remains crucial [22, 23]. Local anti-CD40 Ab administration has been shown to eradicate treated-site tumor and induce a systemic CTL response that eradicates distal tumors [24, 25]. However, the role of CD40-activated CD4+ T cells in helping local versus systemic effector CD8+ T cell function is less well understood. It is possible that anti-CD40 Ab plays a key role in systemic antitumor immunity by releasing not only tumor-specific CTL [25, 26] but also CD4+ T cells, from the dLN into the circulation. One concern is that anti-CD40 Ab treatment may lead to the deletion of tumor-specific CD8+ T cells; however, this response has been rescued by combination with tumor antigen in the form of viral immunization [27].
IL-2 combined with anti-CD40 antibody may represent an alternative rescue strategy by enhancing CD8+ T cell activation [28, 29], preventing anergy of tumor-specific CD8+ T cells [30], and promoting CD8+ T cell proliferation and effector function [31]. Exogenous IL-2 may be critically required for the development and execution of an effective effector/memory response in the tumor vaccine setting [32, 33]. In the absence of sufficient CD4+ T cell help, exogenous IL-2 may re-activate proliferation of CD8+ T cells that have undergone activation-induced non-responsiveness [34]. Furthermore, recent studies have shown that endogenous IL-2 provided by CD4+ T cells during priming is required for the development of functional memory CD8+ T cells [28, 29].
Here, we used a model of murine mesothelioma to examine the key immune mechanisms responsible for eradicating untreated distal tumors and installing T cell memory after use of a local anti-CD40 Ab with or without IL-2 vaccination approach. CD4+ and CD8+ T cell responses in dLNs, non-dLNs, and tumors were monitored, and a two-tumor model and depletion studies were used to assess their role in eradicating distal tumors. Rechallenge plus/minus depletion studies were used to identify the points at which CD4+ and CD8+ memory T cells were generated and required.
Materials and methods
Mice
Female C57BL/6J (H-2b) mice aged 6–8 weeks were obtained from the Animal Resources Centre (Perth, Australia). All mice were used in accordance with institutional guidelines and approval of the UWA and Curtin University’s Animal Ethics Committees (AEC). Mice were injected subcutaneously (s.c.) with 5 × 105 tumor cells per site, and tumor growth monitored. Animals were sacrificed when tumors reached 100 mm2 as per AEC conditions.
Murine tumor cell lines and the murine model
AE17 is a malignant mesothelioma cell line derived from C57BL/6J mice injected with asbestos fibers as previously described [12]. AE17-sOVA was developed by stably transfecting the parental cell line (AE17) with secretory ovalbumin (sOVA; [12]).
CD4+ and CD8+ depletions
For depletion of CD4+ or CD8+ cells, two doses (150 μg/dose) of either YTS-191 or YTS-169 (both from the European Collection of Animal Cell Cultures (ECACC; Salisbury, UK) were injected i.p. before anti-CD40 Ab ± IL-2 treatment and continued (three doses/week; 100–150 μg/dose) as described for each experiment. FACS analysis showed that CD4+ depletion was 90–95% effective and CD8+ cell depletion was 95–99% effective (data not shown).
IL-2 and agonist anti-CD40 Ab
Proleukin (rhIL-2; Cetus Corporation, Emeryville, CA, USA) and anti-CD40 Ab (FGK45; WEHI, Melbourne, Victoria, Australia) were diluted in PBS to the required concentration as previously described [11]. Mice bearing small tumors were treated with the IL-2 and anti-CD40 Ab monotherapies, while mice with large tumors were treated with the IL-2/anti-CD40 Ab combination therapy as previously described [11].
FACS analysis
Samples were prepared as a single-cell suspension and stained for FACS analysis using anti-CD4 (clone RM4-5; Pharmingen) or anti-CD8 (clone 53-6.7; Pharmingen). Analysis was performed on a FACScan (Becton–Dickinson, Mountain View, CA) using Cell Quest software.
Tetramer staining
Single-cell suspensions were sequentially incubated with Fc block (CD16/32; Pharmingen) for 30 min on ice, the SIINFEKL Kb-PE tetramer (kindly donated by Dr Andrew Brooks University of Melbourne, Victoria, Australia) for 1 h at room temperature, and anti-CD8 tricolour (Caltag, Burlingame, CA, USA) combined with anti-CD44-FITC (Pharmingen) for 30 min on ice. FACS analysis was performed on a Becton–Dickinson FACScan using Cell Quest V3.1f Apple Software. Treated and untreated mice with untransfected AE17 tumors were used as the negative control.
In vivo CTL assay
Target cells for in vivo evaluation of cytotoxic activity were prepared as previously described [12]. Briefly, C57BL/6J spleen and LN cell suspensions were divided into two populations. One population was pulsed with 1 × 106 M SIINFEKL for 90 min at 37°C, washed in PBS, and labeled with a high concentration (5 mM) of CFSE (Invitrogen). Control, uncoated target cells were labeled with a low concentration of CFSE (0.5 mM). An equal number of cells from each population were pooled and injected i.v. into recipient mouse (2 × 107 cells total/mouse). Lymph nodes (dLN and non-dLN) from recipient mice were analyzed by flow cytometry 18 h after injection. The ratio between the percentage of uncoated versus SIINFEKL coated was calculated to obtain a numerical value of cytotoxicity. Naïve mice, as well as treated and untreated mice with untransfected AE17 tumors, were used as controls.
Statistical analysis
Statistical significance was calculated using GraphPad PRISM. Student’s t test was used to determine differences between two populations. One-way analysis of variance (ANOVA) was used to determine differences between more than two populations.
Results
CD40 activation broadens CD4+ and CD8+ T cell circulation
The murine mesothelioma AE17 model is one of the few subcutaneous tumor models that is generated by the appropriate carcinogen and maintains human features of the disease [12]. In the first series of studies, a single tumor model was used in which AE17 tumor cells were s.c. inoculated on day 0. Tumors were left to develop into tumors ranging from 25 to 50 mm2 before therapies consisting of three i.t. injections per week for 2 weeks with PBS, IL-2, anti-CD40 Ab, or the IL-2/anti-CD40 Ab combination commenced, as previously described [11].
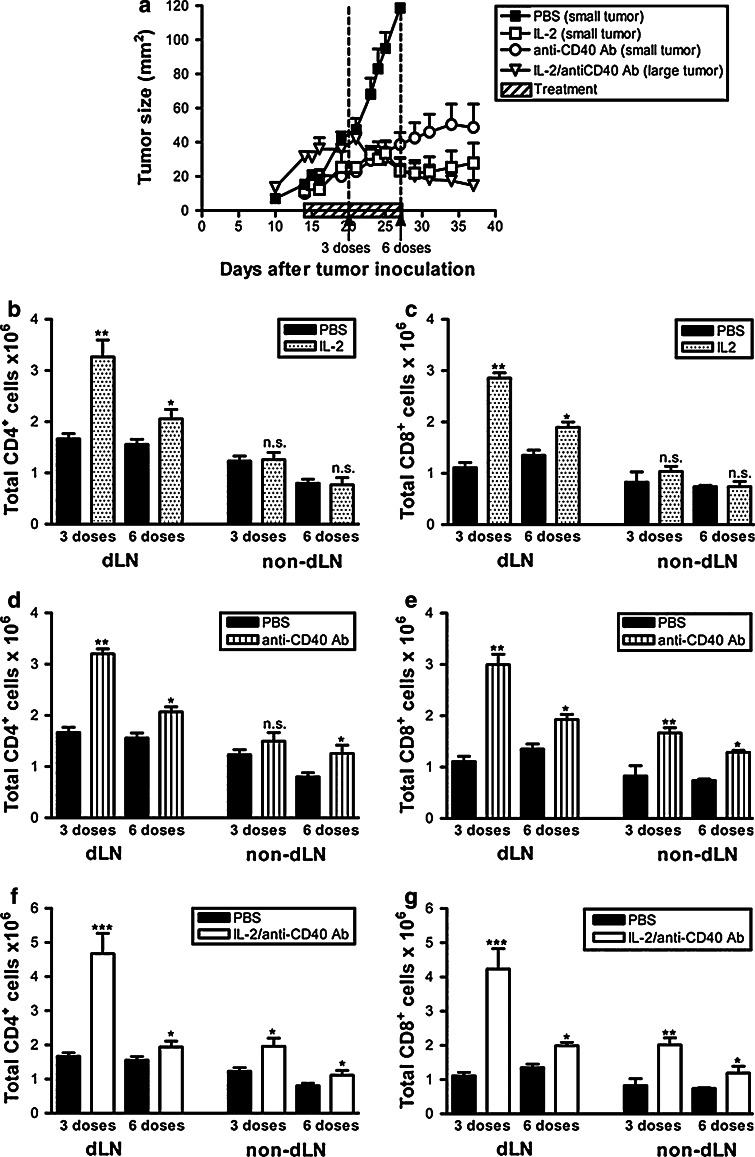
To monitor the strength and extent of the T cell response, dLN and non-dLN were collected after three doses or six doses of treatment (Fig. 1a) and the absolute numbers of CD4+ and CD8+ cells determined. We have previously used the AE17 and AE17-sOVA mesothelioma models to demonstrate that the i.t. IL-2 monotherapy induced CD4+ and CD8+ T cell-dependent regression of treated-site tumors [12]. However, this response had no effect on a distal tumor implanted on the opposite flank [11]. We now postulate that this is because the IL-2-driven changes in the CD4+ and CD8+ T cell response, relative to the diluent control (PBS), were restricted to the dLN (Fig. 1b, c, respectively) and tumor (published in [12]), and no changes were seen in the non-dLN (Fig. 1b, c). These data suggest that i.t. IL-2 induces a local immune response consisting of T cells trafficking between the dLN and tumor, but it does not induce systemic immunity. In contrast, the i.t. anti-CD40 Ab monotherapy induced an increase in CD4+ and CD8+ T cell numbers in draining and non-dLNs (Fig. 1d, e). This was associated with the resolution of treated-site (Figs. 1a, 4a) and distal untreated tumors (Fig. 4b, and [11]), suggesting that CD40 ligation releases activated T cells from the local site enabling access to distal tumors. The CD4+ T cell differences between PBS and anti-CD40 Ab-treated mice in non-dLNs reached statistical difference by the sixth dose, whereas the CD8+ T cell difference was already clear by the third dose. The caveat for both monotherapies is that treatment needs to commence when the tumors are established but small in size and completely fail in larger tumors [11, 12]. In contrast, the IL-2/anti-CD40 Ab combination is effective in larger tumors, and this combination generated a CD4+ and CD8+ T cell response that was already significantly increased in non-dLNs by the third dose (Fig. 1f, g).
Fig. 1.
Anti-CD40 Ab extends the CD4+ and CD8+ cell response to non-dLNs. C57BL/6J mice inoculated with 5 × 105 AE17 tumor cells s.c. on day 0 were left to develop tumors ranging from 16 to 40 mm2 before therapy commenced. Treatment regimens consisted of three i.t. injections per week for 2 weeks with PBS (the diluent control, n = 15 mice), IL-2 (20 μg/dose into small tumors, n = 15 mice) or anti-CD40 Ab (40 μg/dose into small tumors, n = 15 mice) as monotherapies, as well the IL-2/anti-CD40 Ab combination (same doses into large tumors, n = 15 mice: a). Pooled data shown from three experiments (60 mice total) are represented as mean ± SEM. In a separate experiment, AE17 tumor-bearing mice were given three or six i.t. doses of PBS (n = 8 mice), IL-2 (20 μg/dose, n = 8 mice; b, c), anti-CD40 Ab (40 μg/dose, n = 8 mice; d, e), or IL-2/anti-CD40 Ab (n = 10 mice, f, g). Total CD4+ (b, d, f) and CD8+ (c, e, g) numbers from the dLN and non-dLN were calculated by flow cytometry. Pooled data shown from two experiments (42 mice for each timepoint, total mice = 84) are represented as mean + SEM. *P < 0.05, **P < 0.01, ***P < 0.001, n.s. not significant comparing treated groups to PBS treated
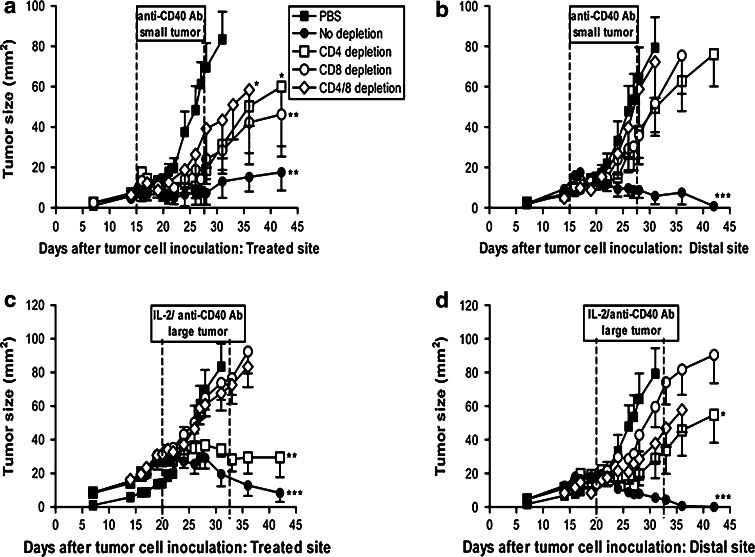
Fig. 4.
CD4+ and CD8+ T cells are critically required for regression of distal tumors. C57BL/6J mice were inoculated with 5 × 105 AE17 tumor cells s.c. into the left and right flanks and treatment commenced into either small (range 1–20 mm2; day 15) or large tumors (range 30–56 mm2; day 20). Two days before the start of i.t. PBS (n = 9 mice), i.t. anti-CD40 Ab (into small tumors, n = 9 mice; a, b) or i.t. IL-2/anti-CD40 Ab (into large tumors, n = 9 mice; c, d) animals were depleted of CD4+ cells and/or CD8+ cells. Depletions were continued for 14–16 days, and cells returned approximately 5 days after the final depleting antibody injection. Pooled data from two experiments (total mice = 90) are shown as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 comparing treatment groups to PBS-treated controls
Anti-CD40 Ab broadens the tumor-specific CD8+ T cell response
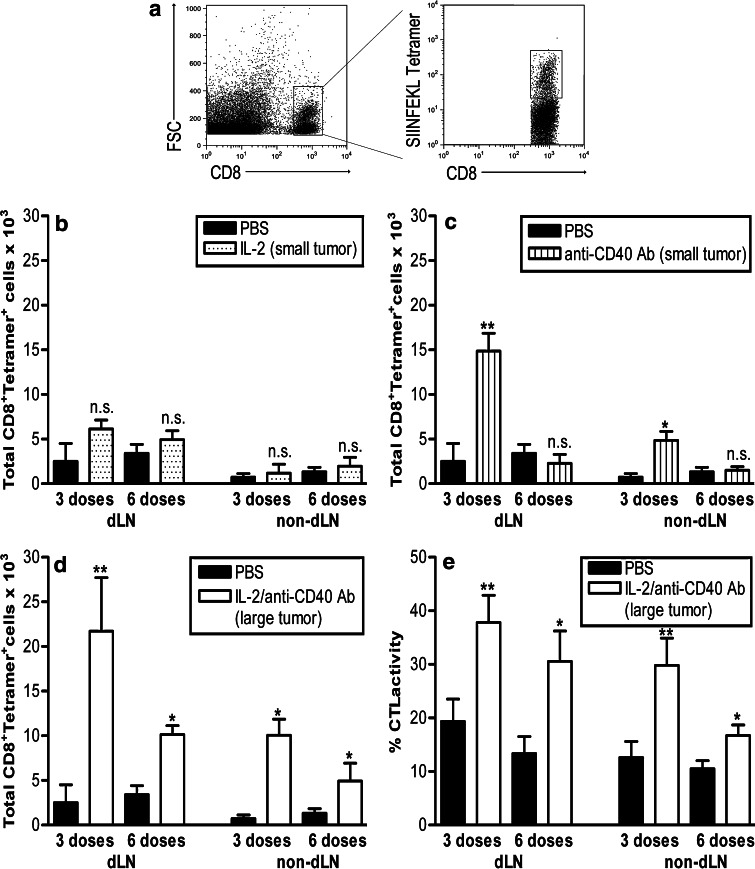
To examine tumor-specific CD8+ T cell responses, mice bearing the OVA transfectant (AE17-sOVA), in which OVA becomes a marker tumor antigen, were also treated with IL-2, anti-CD40 Ab, or both using the same approach described earlier. OVA-specific MHC class I tetramer staining allowed enumeration of tumor antigen-specific CD8+ T cells (Fig. 2a). Examination of dLNs showed that OVA-tetramer+CD8+ T cell numbers dramatically and significantly increased after three doses with anti-CD40 Ab alone or the IL-2/anti-CD40 Ab combination relative to IL-2 and PBS-treated mice (Fig. 2b–d). After six doses, tumor-specific CD8+ T cells numbers had declined, although mice treated with IL-2/anti-CD40 Ab displayed significantly higher numbers than the other groups. A similar trend, albeit with lower overall numbers of tetramer+CD8+ T cells, was seen in the non-dLN, and once again the response persisted longer in mice treated with IL-2/anti-CD40 Ab (Fig. 2b–d). Note that the IL-2/anti-CD40 Ab-treated mice (Fig. 2d) demonstrated an amplified tumor-specific CD8+ T cell response that was twice the size of the anti-CD40 Ab monotherapy (Fig. 2c). These data imply that IL-2 promotes CD40-activated T cell proliferation, survival, and function.
Fig. 2.
IL-2 amplifies and prolongs CD40-driven tumor-specific CD8+ T cell responses. C57BL/6J mice bearing small (9–20 mm2) or large AE17-sOVA tumors (25–42 mm2) were given three or six i.t. doses of PBS (n = 8 mice), IL-2 (small tumors, n = 8 mice; b), anti-CD40 Ab (small tumors, n = 8 mice; c), or IL-2/anti-CD40 Ab (large tumors, n = 10 mice; d). The dLNs and non-dLNs were prepared as a single suspensions, stained for OVA-tetramer+CD8+ cells (gating strategy shown in a), and total cell numbers calculated by flow cytometry. Fluorescently labeled, target cells expressing MHC class I bound SIINFEKL were adoptively transferred into tumor-bearing mice treated with three or six i.t. doses of IL-2/anti-CD40 Ab (e), and in vivo CTL activity was determined in the dLN and non-dLN. Pooled data shown from two experiments (42 mice for each timepoint, total mice = 84) are represented as mean ± SEM. **P < 0.01, *P < 0.05, n.s. not significant comparing treated groups to PBS-treated controls
We have previously shown that IL-2 increased CTL activity in animals bearing small but not large tumors [12]. In contrast, anti-CD40 antibody alone did not increase CTL activity ([13] and Suppl Fig 1). Therefore, we next examined in vivo CTL activity in lymph nodes of large AE17-sOVA tumor-bearing mice after IL-2/anti-CD40 Ab (Fig. 2e). IL-2/anti-CD40 antibody treatment significantly enhanced tumor-specific CTL activity after three and six doses compared with PBS-treated controls (Fig. 2e and data not shown). These data suggest that IL-2 enhances the quality (i.e., increased CTL activity), but not the quantity of T cells (as shown by the tetramer data in Fig. 2b). In contrast, anti-CD40 Ab increases the number (Fig. 2c) but not the activity of tumor-specific T cells [13]. The IL-2/anti-CD40 Ab combination induces a large, prolonged, and highly active antitumor-specific response (Fig. 2d, e).
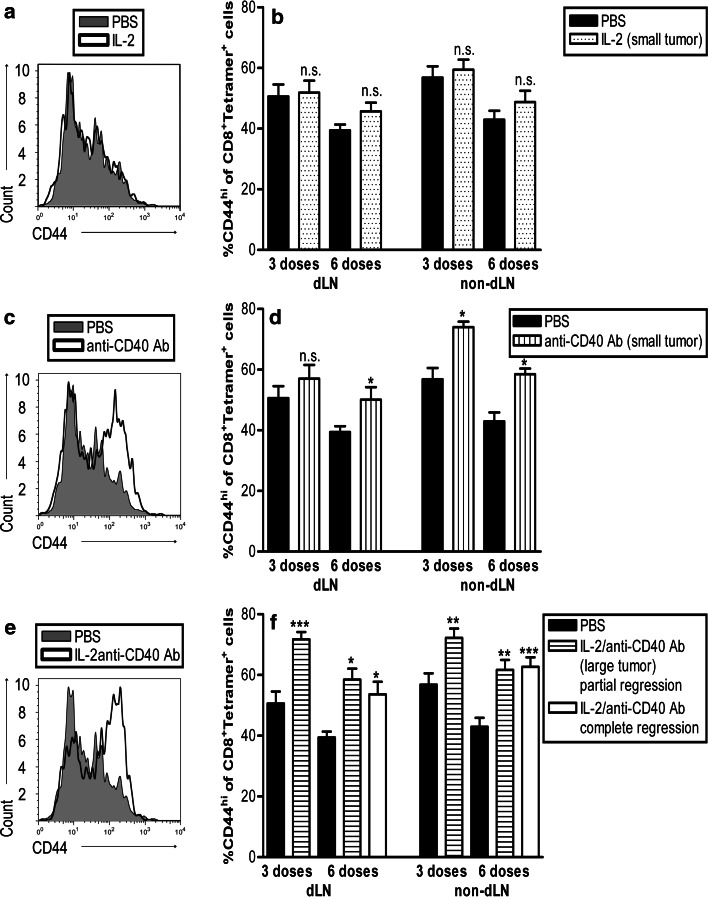
Anti-CD40 Ab drives tumor-specific T cells to express high levels of CD44
CD44 is upregulated on lymphocytes following activation and remains high thereafter [35]. High levels of CD44 are indicative of effector/memory cells as CD44 is important for survival during clonal expansion and subsequent entry into the memory compartment [36]. Tetramer+CD8+ cells in the dLN and non-dLN of IL-2-treated mice did not exhibit increased CD44 compared with PBS controls (Fig. 3a, b). In contrast, CD44 expression was increased in anti-CD40 Ab-treated mice (Fig. 3c, d). This was more pronounced in IL-2/anti-CD40 Ab-treated mice as CD44 expression on tetramer+CD8+ cells in the dLN and non-dLN peaked at three doses and remained high at six doses (Fig. 3e, f). This was also observed in mice with complete tumor resolution after six doses (Fig. 3f). These data suggest that IL-2/CD40-driven tumor-specific CD8+ T cells may be transitioning to effector/memory cells.
Fig. 3.
CD40 drives activated CD44hi tumor-specific CD8+ T cells. C57BL/6J mice bearing small (9–20 mm2) or large AE17-sOVA tumors (25–42 mm2) were given three or six i.t. doses of PBS (n = 8 mice), IL-2 (into small tumors, n = 8 mice; a, b), anti-CD40 Ab (into small tumors, n = 8 mice; c, d), or IL-2/anti-CD40 Ab (into large tumors, n = 10 mice; e, f). The dLNs and non-dLNs were prepared as single suspensions and stained for CD44, OVA-tetramer, and CD8. The percentage of OVA-tetramer+CD8+ cells that were CD44hi was calculated by flow cytometry. Representative histograms of CD44 staining from IL-2-treated (a), anti-CD40 Ab-treated (c) and IL-2/anti-CD40 Ab treated (e) from the dLN are shown after 6 doses. Pooled data (b, d, f) are shown from two experiments (42 mice for each timepoint, total mice = 84) represented as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05, n.s. not significant comparing treated groups to PBS-treated controls
Eradicating distal untreated tumors requires CD4+/CD8+ T cell collaboration
The next series of experiments addressed the role T cells play in eradicating distal untreated tumors using a two-tumor model. To do this, mice inoculated with AE17 cells s.c. into both the left and right flanks on day 0 were separated into those with small or large tumors. Two days before the start of anti-CD40 Ab ± IL-2 treatment, animals were depleted of CD4+ T cells, CD8+ T cells, or both. Depletions continued throughout the treatment period; generally, the depleted cells returned 5 days after the last dose. IL-2 was not included as we have previously shown that IL-2 does not impact on untreated distal tumors [11].
Anti-CD40 Ab alone inhibited the treated-site tumor growth in 70% of mice, and mice depleted of CD4+ and/or CD8+ T cells maintained a partial antitumor response after anti-CD40 Ab (Fig. 4a). Interestingly, the contralateral untreated tumors continued to grow at almost the same rate as PBS-treated controls in the absence of CD4+ and/or CD8+ T cells (Fig. 4b). These data imply that both T cell populations are critically required for the regression of distal tumors, yet only partially responsible for the resolution of treated-site tumors after anti-CD40 Ab treatment.
Immunologically intact (i.e., no depletion) mice responded to the combination treatment of IL-2/anti-CD40 Ab into both small (data not shown) and large tumors leading to the regression of treated-site and untreated distal tumors (Fig. 4c, d). Treatment of large tumors with IL-2/anti-CD40 Ab remained effective in the treated-site tumor in CD4+ depleted animals, but not in mice depleted of CD8+ or both CD4+ and CD8+ (Fig. 4c). These data clearly show that IL-2/anti-CD40 Ab-driven treated-site tumor eradication is critically dependent on CD8+ but not CD4+ T cells. Once again, the contralateral tumor responded differently as depletion of CD4+, CD8+ cells, or both led to their continued growth (Fig. 4d). Similar to the anti-CD40 Ab monotherapy data, these data imply that while CD4+ cells are not required for treated-site tumor eradication, they are critically required, in association with CD8+ T cells, for destruction of distal site untreated tumors.
CD40/IL-2-activated CD4+ and CD8+ T cells are necessary and sufficient for protective memory
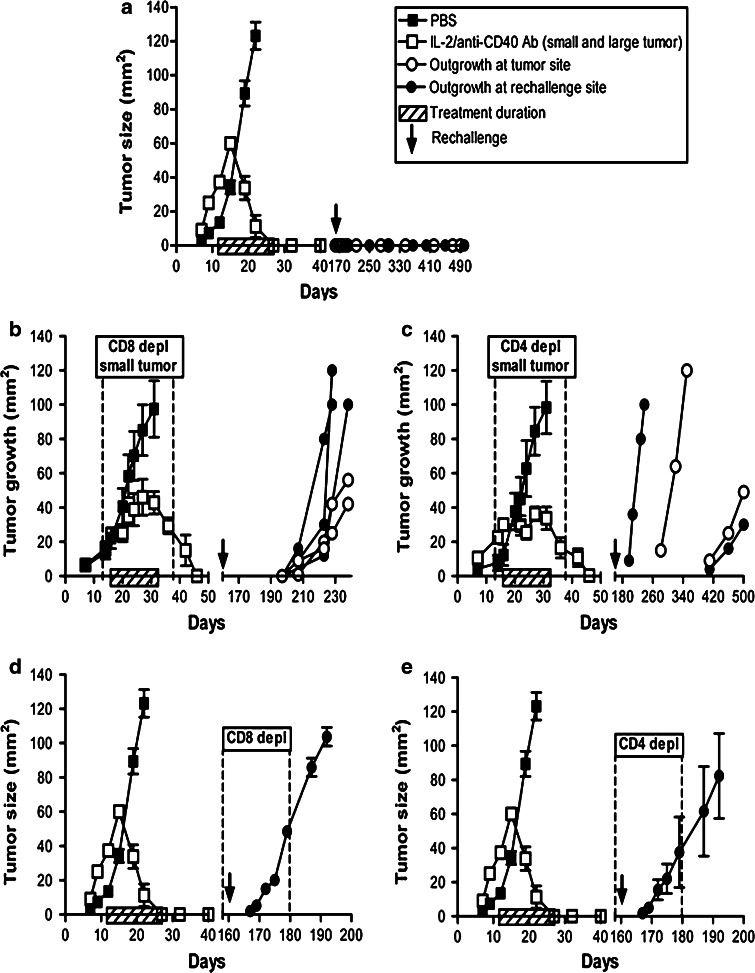
Mice bearing single AE17 or AE17-sOVA tumors that had completely regressed after the IL-2 or anti-CD40 Ab monotherapies or the IL-2/anti-CD40 Ab combination were left for varying periods of time ranging from two to 12 months and then rechallenged with 5 × 105 AE17 or AE17-sOVA cells at either the original tumor implantation site or the contralateral site as shown in Table 1. All treatment groups resulted in the majority of animals protected regardless of the site of rechallenge. Interestingly, while IL-2 was unable to remove untreated distal tumors, IL-2 provided protection to 79% of mice. This indicates that IL-2 is capable of inducing a protective memory response. In contrast, all mice treated with anti-CD40 Ab ± IL-2-treated mice were protected. Interestingly, mice with AE17-sOVA as their original treated tumor were protected from AE17 tumors, suggesting that OVA is not the dominant tumor antigen in this model. Mice protected from rechallenge were challenged with AE17 tumor cells yet again at another site (data not shown). All mice remained tumor-free for the remainder of their natural lives (>500 days), confirming that each treatment induces protective immunological memory.
Table 1.
All treatments install immunological memory
| Treatment | Original tumor | Rechallenge | Rechallenge site | Number protected |
|---|---|---|---|---|
| IL-2 | AE17 | AE17 | Tumor site | 9/10 |
| IL-2 | AE17-sOVA | AE17 | Tumor site | 4/6 |
| IL-2 | AE17-sOVA | AE17-sOVA | Tumor site | 3/4 |
| IL-2 | AE17 | AE17 | Contralateral | 10/12 |
| IL-2 | AE17-sOVA | AE17 | Contralateral | 4/6 |
| anti-CD40 Ab | AE17 | AE17 | Tumor site | 4/4 |
| anti-CD40 Ab | AE17 | AE17 | Contralateral | 3/3 |
| IL-2/anti-CD40 Ab | AE17 | AE17 | Tumor site | 13/13 |
| IL-2/anti-CD40 Ab | AE17-sOVA | AE17 | Tumor site | 6/6 |
| IL-2/anti-CD40 Ab | AE17 | AE17 | Contralateral | 9/9 |
| IL-2/anti-CD40 Ab | AE17-sOVA | AE17 | Contralateral | 4/4 |
C57BL/6J mice inoculated with 5 × 105 AE17 tumor cells s.c. on day 0 were left to develop tumors ranging from 16 to 40 mm2 before therapy commenced. Treatment regimens consisted of three i.t. injections per week for 2 weeks with PBS (the diluent control), IL-2 (20 μg/dose into small tumors) or anti-CD40 Ab (40 μg/dose into small tumors) as monotherapies, as well the IL-2/anti-CD40 Ab combination (into large tumors). Mice with complete tumor regression after treatment were left for varying periods of time ranging from two to 12 months and then rechallenged with 5 × 105 AE17 or AE17-sOVA cells at either the original tumor implantation site or at the contralateral site. Shown are the number of mice protected from the total that were rechallenged in each of the different treatment groups
The next series of experiments focussed on the IL-2/anti-CD40 Ab combination therapy as it induced resolution of large tumors [11] and provided 100% immunological protection. We have previously demonstrated that a number of mice treated with IL-2/anti-CD40 Ab completely eradicated their tumors even in the absence of CD4+ or CD8+ cells during therapy (68% of CD4+ depleted mice and 53% of CD8+ depleted mice; see Figs. 2 and 3 in [11]). Therefore, to further examine the role of CD4+ or CD8+ cells in long-term memory responses, completely cured mice were rechallenged with AE17 cells contralateral to the original tumor site. Mice that had been immunologically intact during therapy were completely protected and no tumors emerged (Fig. 5a). In contrast, tumors emerged at varying times from two to 12 months post-rechallenge in 56% of animals that had been depleted of either CD8+ or CD4+ cells during therapy (Fig. 5b, c). Interestingly, in a few animals depleted of CD8+ (Fig. 5b) or CD4+ (Fig. 5c) cells during therapy, tumors appeared at the original site and not the rechallenge (or contralateral) site; this occurred up to 12 months after being ‘cured’ of the original tumor. To the best of our knowledge, this is one of the few studies to examine mice for the duration of their natural lives. These data show that IL-2/CD40 activation of both CD4+ and CD8+ T cells is required for the generation of effective immunological memory and tumor surveillance.
Fig. 5.
CD4+ and CD8+ T cells are critically required for the development of effective memory. C57BL/6J mice bearing large AE17 tumors were depleted of either CD4+ cells or CD8+ cells 2 days before the start of IL-2/anti-CD40 Ab treatment (small and large tumors). Depletions were continued for 14–16 days, and cells returned approximately 5 days after final depleting antibody injection. Mice exhibiting complete tumor regression for at least 3 months were rechallenged with 5 × 105 AE17 tumor cells (16 mice total). Mice with no depletion during the primary or effector memory response were rechallenged as a positive control (n = 8 mice/group; a). Naïve C57BL/6J mice were inoculated with AE17 tumor cells at the same time as the rechallenge mice to confirm the tumor cell preparation, these grew at the same rate as PBS in a (data not shown). Individual mice are represented for mice treated with IL-2/anti-CD40 Ab and CD8+ depleted (n = 5 mice; b), or CD4+ depleted (n = 4 mice; c). In another experiment, C57BL/6J mice previously cured with IL-2/anti-CD40 Ab (no depletion during treatment) were depleted of either CD8+ (d, n = 5 mice/group) or CD4+ cells (e, n = 6 mice/group) 2 days before rechallenge with 5 × 105 AE17 tumor cells contralateral to the original tumor site. Depletions were continued for 16–18 days. Data from one experiment are shown as mean ± SEM. Naïve C57BL/6J mice were inoculated with AE17 tumor cells at the same time as the rechallenge mice to confirm the tumor cell preparation, and these grew at the same rate as PBS (data not shown)
To further examine the role of CD4+ and CD8+ cells in the generation of memory, animals previously cured with IL-2/anti-CD40 Ab, and not T cell depleted during treatment, were depleted of either CD4+ or CD8+ T cells 2 days prior to tumor cell rechallenge with 5 × 105 AE17 tumor cells on the contralateral site. Depletion continued until emerging tumors reached 40 mm2. All animals (5/5 CD8+ depleted and 6/6 CD4+ depleted) developed AE17 tumors at the same rate as the control mice (Fig. 5d, e). In contrast, immunologically intact mice at the time of rechallenge were completely protected (Fig. 5a). Depletion of CD4+ or CD8+ cells did not lead to tumor recurrence at the local treated-site. Taken together, these data confirm that CD4+ and CD8+ T cells previously activated via IL-2/CD40 therapy are critically required to protect against rechallenge.
Discussion
In this study, we aimed to identify the key T cell effector mechanisms required to eradicate inaccessible, untreatable tumor deposits using a two-tumor model system, in which one tumor was treated with locally applied IL-2 and/or agonist anti-CD40 Ab, while the other distal tumor was left untouched. We report that different effector mechanisms can operate to eradicate treated-site versus distal tumors, i.e., when agonist anti-CD40 Ab is combined with IL-2 the local effector response bypasses CD4+ help, however, collaborating CD4+ and CD8+ T cells were critically required for eradicating untreated distal tumors and for long-term protection. These data suggest that treatment-dependent responses generated within tumors and dLN determine whether tumor-specific, effector/memory T cells are disseminated from dLNs to patrol the entire body seeking other tumors.
In our model, IL-2 induced a CD4+ and CD8+ T cell-dependent response that led to regression of treated-site tumors but had no effect on distal tumors. This may be explained by our observations suggesting the generation of a T cell response that was limited to the dLN and treated-site tumor, i.e., T cells appeared to be circulating between the dLN and tumor. Furthermore, tetramer+CD8+ T cells did not express increased levels of CD44 (compared with PBS controls), suggesting that IL-2 did not increase the ability of cells to migrate to distant sites. Interestingly, despite the inability of IL-2 to induce a systemic effector response during and immediately after the treatment period, IL-2 treatment still induced an effective long-term systemic memory response. These data differ to a report showing that high doses of locally administered IL-2 induce effector memory T cells, which were site-specific, rather than central memory T cells [37]. Furthermore, others have shown that local IL-2 can induce a systemic response leading to the destruction of distant metastases [32, 33, 38]. The differences may be dose- or model-related. This mesothelioma model grows slowly relative to many other subcutaneous models, and the IL-2 dose used is low.
The local antitumor response induced by agonist anti-CD40 Ab involved B cells and CD8+ T cells, but not CD4+ T cells [13]. However, increased CD4+ and CD8+ T cell numbers were seen in the dLNs and non-dLNs, suggesting that the T cell circuit had been broadened enabling access to, and resolution of, distal untreated tumors. This was further confirmed by an increased expression of CD44 on tetramer+CD8+ T cells within the non-dLN during anti-CD40Ab therapy. Other studies have shown that anti-CD40 Ab may not necessarily increase the number of CD8+ T cells but rather release tumor-specific CTLs from the dLN into the circulation and tumors [25, 26]. Despite the CD40-driven CD4+ T cell response in non-dLNs being slower than CD8+ T cells, including tetramer+CD8+ T cells, CD4+ T cells were still critically required for eradicating distal tumors. It is possible that once these CD4+ T cells have infiltrated a distal tumor, they play a key role in recruiting CD8+ T cells [31].
Combining IL-2 with anti-CD40 Ab induced acute intratumoral inflammation [11] involving a large number of neutrophils and CD8+ T cells. In this study, we extended these studies and showed that IL-2/anti-CD40 Ab treatment led to high expression of CD44 on tumor-specific CD8+ T cells, a marker that is critical for trafficking of lymphocytes during inflammation [39]. Inducing local inflammation may be a critical event that activates key cell types including APC such as DCs and effector T cells that traffic between dLN and tumor, and beyond as the response for CD4+, CD8+, tetramer+CD8+ T cells and in vivo CTL activity in non-dLNs was rapid, and regardless of their size distal tumors regressed. Furthermore, inflammatory signals provided by cytokines such as type I interferons and/or IL-12 are essential for normal effector and memory CTL generation [40].
We also assessed the ability of the different immunotherapies to install T cell memory. The two monotherapies and the anti-CD40 Ab/IL-2 combination all generated a memory response and induced enlargement of local lymph nodes [11–13]. Recent work has shown that enlarged lymph nodes during the priming phase enhance secondary responses by functioning as a depot for memory cells [41]. CD40 signaling may release these cells into the circulation [25, 26].
The role of CD4+ T cell help in effector/memory CD8+ T cell generation in tumor vaccination strategies is unclear as most effector/memory studies involve viral immunization models. The development of memory CD8+ responses is reported to diminish without CD4+ T cell help [22, 23], while others have shown that regardless of CD40 activation, CD4+ T cell help for CD8+ T cells remains crucial [42, 43]. Our studies show that the generation of an effective memory response required the presence both CD4+ and CD8+ T cells during therapy and during the effector/memory phase. Similarly, our depletion studies showed that CD4+ T cells and CD8+ T cells had to be present during IL-2/CD40-driven activation (therapy) for the generation and execution of a protective memory response. Therefore, in our model, agonist anti-CD40 Ab treatment did not bypass the need for CD4+ T cell help during the effector/memory phase, as suggested by others [21]. Furthermore, mice that were cured with IL-2/anti-CD40 Ab maintained high expression of CD44 on tumor-specific CD8+ T cells, suggesting that these cells were transitioning into effector memory cells. However, further studies are required to address this issue.
In summary, our novel findings are that local three treatment regimens using IL-2 and/or agonist anti-CD40 Ab each generate different effector mechanisms for the eradication of treated-site versus untreated distal tumors. The use of agonist anti-CD40 Ab with or without IL-2 induced a local response that was CD4+ independent. In contrast, even with exogenous CD40 signaling, CD4+/CD8+ T cell collaboration was critically required for the eradication of untreated distal tumors and for the installation and execution of effector/memory responses. In these studies, local i.t. treatment was used to modulate the mesothelioma tumor microenvironment; other reagents may elicit a similar response in this and other models [44, 45]. Generating local inflammation via IL-2/anti-CD40 Ab therapy resulted in collaborating CD4+ and CD8+ T cells that not only patrolled the whole body but penetrated and eradicated distal untreated tumors and protected from rechallenge. Tumor-targeting strategies such as CT-guided injections, nanotechnology, monoclonal antibodies, and gene therapy are increasingly being used in the clinical setting. Combined with our results, these rapid advances in technology support the development of local therapies for the improved treatment of solid tumors, including malignant mesothelioma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Cancer Council of WA, the Mesothelioma Applied Research Foundation (MARF), and the Australian Lung Foundation (ALF).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- Ab
Antibody
- i.t.
Intratumoral
- dLN
Draining lymph node
- non-dLN
Non-draining lymph node
References
- 1.Hargadon KM, Brinkman CC, Sheasley-O’neill SL, Nichols LA, Bullock TN, Engelhard VH. Incomplete differentiation of antigen-specific CD8 T cells in tumor-draining lymph nodes. J Immunol. 2006;177:6081–6090. doi: 10.4049/jimmunol.177.9.6081. [DOI] [PubMed] [Google Scholar]
- 2.Nelson DJ, Mukherjee S, Bundell C, Fisher S, van Hagen D, Robinson B. Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J Immunol. 2001;166:5557–5566. doi: 10.4049/jimmunol.166.9.5557. [DOI] [PubMed] [Google Scholar]
- 3.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via cross presentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/S1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson ED, Enriquez HL, Fu YX, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207:1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB. CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol. 2001;167:5042–5051. doi: 10.4049/jimmunol.167.9.5042. [DOI] [PubMed] [Google Scholar]
- 6.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64:2865–2873. doi: 10.1158/0008-5472.CAN-03-3066. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Hao S, Li F, Ye Z, Yang J, Xiang J. CD4+ Th1 cells promote CD8+ Tc1 cell survival, memory response, tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007;120:148–159. doi: 10.1111/j.1365-2567.2006.02452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorne SH, Liang W, Sampath P, Schmidt T, Sikorski R, Beilhack A, et al. Targeting localized immune suppression within the tumor through repeat cycles of immune cell-oncolytic virus combination therapy. Mol Ther. 2010;18:1698–1705. doi: 10.1038/mt.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosendahl A, Kristensson K, Carlsson M, Skartved NJ, Riesbeck K, Sogaard M, et al. Long-term survival and complete cures of B16 melanoma-carrying animals after therapy with tumor-targeted IL-2 and SEA. Int J Cancer. 1999;81:156–163. doi: 10.1002/(SICI)1097-0215(19990331)81:1<156::AID-IJC25>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, Bose A, Komita H, Taylor JL, Kawabe M, Chi N, et al. Intratumoral IL-12 gene therapy results in the crosspriming of Tc1 cells reactive against tumor-associated stromal antigens. Mol Ther. 2010;19:805–814. doi: 10.1038/mt.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, et al. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20:1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 12.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, et al. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 13.Jackaman C, Cornwall S, Graham PT, Nelson DJ. CD40-activated B cells contribute to mesothelioma tumor regression. Immunol Cell Biol. 2011;89:255–267. doi: 10.1038/icb.2010.88. [DOI] [PubMed] [Google Scholar]
- 14.Sterman DH, Haas A, Moon E, Recio A, Schwed D, Vachani A et al (2011) A Trial of intrapleural adenoviral-mediated interferon-{alpha}2b gene transfer for malignant pleural mesothelioma. Am J Respir Crit Care Med. doi:10.1164/rccm.201103-0554CR [DOI] [PMC free article] [PubMed]
- 15.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 16.Bazan-Peregrino M, Seymour LW, Harris AL. Gene therapy targeting to tumor endothelium. Cancer Gene Ther. 2007;14:117–127. doi: 10.1038/sj.cgt.7701001. [DOI] [PubMed] [Google Scholar]
- 17.Gerber HP, Senter PD, Grewal IS. Antibody drug-conjugates targeting the tumor vasculature: current and future developments. MAbs. 2009;1:247–253. doi: 10.4161/mabs.1.3.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 19.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 20.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 21.Tutt AL, O’Brien L, Hussain A, Crowther GR, French RR, Glennie MJ. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168:2720–2728. doi: 10.4049/jimmunol.168.6.2720. [DOI] [PubMed] [Google Scholar]
- 22.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Herrath MG, Yokoyama M, Dockter J, Oldstone MB, Whitton JL. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J Virol. 1996;70:1072–1079. doi: 10.1128/jvi.70.2.1072-1079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res. 2011;17:2270–2280. doi: 10.1158/1078-0432.CCR-10-2888. [DOI] [PubMed] [Google Scholar]
- 25.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, et al. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol. 2004;173:5923–5928. doi: 10.4049/jimmunol.173.10.5923. [DOI] [PubMed] [Google Scholar]
- 27.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc Natl Acad Sci USA. 2001;98:10811–10816. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai YP, Lin CC, Liao WJ, Tang CY, Chen SC. CD4+ T cell-derived IL-2 signals during early priming advances primary CD8+ T cell responses. PLoS One. 2009;4:e7766. doi: 10.1371/journal.pone.0007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrikant P, Mescher MF. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J Immunol. 1999;162:2858–2866. [PubMed] [Google Scholar]
- 31.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maas RA, Dullens HF, De Jong WH, Den Otter W. Immunotherapy of mice with a large burden of disseminated lymphoma with low-dose interleukin 2. Cancer Res. 1989;49:7037–7040. [PubMed] [Google Scholar]
- 33.Vaage J. Peri-tumor interleukin-2 causes systemic therapeutic effect via interferon-gamma induction. Int J Cancer. 1991;49:598–600. doi: 10.1002/ijc.2910490422. [DOI] [PubMed] [Google Scholar]
- 34.Shrikant P, Mescher MF. Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J Immunol. 2002;169:1753–1759. doi: 10.4049/jimmunol.169.4.1753. [DOI] [PubMed] [Google Scholar]
- 35.Huet S, Groux H, Caillou B, Valentin H, Prieur AM, Bernard A. CD44 contributes to T cell activation. J Immunol. 1989;143:798–801. [PubMed] [Google Scholar]
- 36.Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrama D, Xiang R, Eggert AO, Andersen MH, Pedersen Ls LO, Kampgen E, et al. Shift from systemic to site-specific memory by tumor-targeted IL-2. J Immunol. 2004;172:5843–5850. doi: 10.4049/jimmunol.172.10.5843. [DOI] [PubMed] [Google Scholar]
- 38.Van Es RJ, Baselmans AH, Koten JW, Van Dijk JE, Koole R, Den Otter W. Perilesional IL-2 treatment of a VX2 head-and-neck cancer model can induce a systemic anti-tumour activity. Anticancer Res. 2000;20:4163–4170. [PubMed] [Google Scholar]
- 39.Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208–220. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- 40.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 41.Fransen MF, van Stipdonk MJ, Sluijter M, Schoenberger SP, Melief CJ, Offringa R. Separate roles for antigen recognition and lymph node inflammation in CD8+ memory T cell formation. J Immunol. 2010;185:3167–3173. doi: 10.4049/jimmunol.0904046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J Exp Med. 2003;198:1759–1764. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 44.Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, et al. CCL2 blockade augments cancer immunotherapy. Cancer Res. 2010;70:109–118. doi: 10.1158/0008-5472.CAN-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackaman C, Nelson DJ. Cytokine-armed vaccinia virus infects the mesothelioma tumor microenvironment to overcome immune tolerance and mediate tumor resolution. Cancer Gene Ther. 2010;17:429–440. doi: 10.1038/cgt.2009.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.