Abstract
The primary immune role of B cells is to produce antibodies, but they can also influence T cell function via antigen presentation and, in some contexts, immune regulation. Whether their roles in tumour immunity are similar to those in other chronic immune responses such as autoimmunity and chronic infection, where both pro- and anti-inflammatory roles have been described, remains controversial. Many studies have aimed to define the role of B cells in antitumor immune responses, but despite this considerable body of work, it is not yet possible to predict how they will affect immunity to any given tumour. In many human cancers, the presence of tumour-infiltrating B cells and tumour-reactive antibodies correlates with extended patient survival, and this clinical observation is supported by data from some animal models. On the other hand, T cell responses can be adversely affected by B cell production of immunoregulatory cytokines, a phenomenon that has been demonstrated in humans and in animal models. The isotype and concentration of tumour-reactive antibodies may also influence tumour progression. Recruitment of B cells into tumours may directly reflect the subtype and strength of the anti-tumour T cell response. As the response becomes chronic, B cells may attenuate T cell responses in an attempt to decrease host damage, similar to their described role in chronic infection and autoimmunity. Understanding how B cell responses in cancer are related to the effectiveness of the overall anti-tumour response is likely to aid in the development of new therapeutic interventions against cancer.
Keywords: B cells, Antibodies, Tumour models, Clinical correlations
Clinical evidence
Correlations between the presence of tumour-infiltrating B cells and patient survival have been extensively reviewed recently [1], and only a brief summary will be provided here. Most studies demonstrate a positive association. It is not clear from the clinical observations if this association is specifically related to beneficial effects of B cell functions such as antigen presentation, antibody production, or a combination of both. B cells may also be recruited into tumours in response to IFN-γ production [2, 3] and thus serve as a surrogate marker of an effective T cell-mediated anti-tumour response.
Associations between B cell infiltration and patient survival
Immunohistochemical analysis has indicated that B cell density in tumours and/or lymph node metastases correlates with an improved prognosis in melanoma [4], head and neck cancer [5] and ovarian cancer [6]. In general, fewer B cells are present in visceral metastases [7]. Interestingly, CD20+ tumour-infiltrating lymphocytes (TILs) are often colocalised with CD8+ TILs, which may reflect a microenvironment rich in IFN-γ, recently reported to serve as a B cell tissue attractant via upregulation of CXC chemokine receptor 3 (CXCR3) [2] and/or vascular cell adhesion protein 1 (VCAM-1) [3]. This interpretation is consistent with the improved prognosis for ovarian cancer infiltrated with both B and T cells, compared with T cells alone [6] and the positive effects of intratumoural injections of recombinant human IL-12, a driver of IFN-γ production and switching to Th1-associated serum antibodies such as human IgG1 [8]. An alternative explanation is that the presence of CD4+ and CD8+ T cells and B cells, often organised into tertiary lymphoid structures, reflects the chronic nature of the anti-tumour immune response, and is analogous to that seen in chronic infectious and autoimmune diseases [9]. This interpretation is supported by the oligoclonal nature of B cell infiltrates and the presence of isotype switched, somatically mutated Ig transcripts, suggesting that tumour-infiltrating B cells have been selected for specific recognition of tumour antigens [6].
Bioinformatic analysis quantifying transcripts associated with B cells has proven effective in predicting prognosis in some breast cancer subtypes [10]. Thus, the combination of a “high B cell and low IL-8 gene signature” derived from a metagene expression prediction analysis was associated with a good prognosis in a subgroup of triple negative breast cancer patients [10]. B cell gene expression signatures identified using mRNA sequencing in primary breast cancer tissue also correlated with increased metastasis-free and progression-free survival in basal-like and HER2-enriched breast cancer, as well as in immunoreactive ovarian cancer [11]. In another study, expression of the immunoglobulin kappa C gene was as effective as the entire B cell metagene in predicting improved metastasis-free survival in a large series of breast, non-small cell lung carcinoma and colorectal cancer patients [12]. In cases in which B cell receptor or antibody diversity was examined, the presence of isotype switching and somatic hypermutation indicated that B cells within the tumour microenvironment had received CD4+ T cell help [6, 11]. Taken together, these studies suggest that B cell involvement in the tumour response is a positive clinical indicator, either as a reflection of a type I T cell response, or via intrinsic B cell functions.
Effects of B cell depletion
While B cell-depleting antibodies such as rituximab are commonly used as cytotoxic agents in human B cell-related malignancies, few studies have targeted human B cells as a means of modulating the immune response against non-B cell tumours. Studies in renal cell carcinoma, melanoma [13] and colorectal carcinoma [14] reported no major clinical benefit. In a study in which B cells were depleted as an adjunct to chemotherapy for B cell lymphoma, overall survival was increased despite a concurrent increase in the rate of secondary solid tumour development in B cell-depleted patients [15]. The long-term effects of B cell depletion remain to be established definitively.
Mouse models examining the role of B cells in anti-tumour immunity
T cell anti-tumour responses in B cell-deficient mice (Table 1)
Table 1.
Enhanced anti-tumour responses in B cell-deficient mice
| Role of B cells | Model | Type of deficiency | Immune response | References |
|---|---|---|---|---|
| Negative | T-10 fibrosarcoma | Chronic depletion | Spontaneous | [16] |
| Negative | MCA-induced carcinogenesis | Chronic depletion | Spontaneous | [17] |
| Negative |
TS/A mammary carcinoma J558L plasmacytoma CMS-5 fibrosarcoma |
Genetic knockout | Vaccinated with irradiated tumour cells | [18] |
| Negative | B16 melanoma | Genetic knockout | Vaccinated with adenovirus expressing tumour antigen | [19] |
| Negative |
EL4 thymoma MC38 colon carcinoma B16 melanoma |
Genetic knockout | Spontaneous | [20] |
| Negative |
EL4 thymoma MC38 colon carcinoma EMT6 breast carcinoma |
Genetic knockout | Spontaneous | [21] |
| Negative | EMT-6 breast carcinoma | Genetic knockout | Spontaneous | [22] |
| Negative |
D5 melanoma EL4gag thymoma |
Genetic knockout | Spontaneous | [23] |
| Negative | PDSC5 and WDSC SCC lines | Genetic knockout | Spontaneous | [24] |
Studies conducted in B cell-deficient mice have generally suggested that B cells inhibit rather than enhance spontaneous anti-tumour immunity. Early studies using chronic administration of anti-mouse IgM to deplete B cells reported suppression of the growth of subcutaneous methylcholanthrene (MCA)-induced T-10 tumour cells [16], as well as a longer time to tumour development in the MCA-induced skin carcinogenesis model [17]. Subsequent studies using genetically manipulated B cell-deficient mice such as μMT−/− (also known as Igh-6−/−) and JH−/− confirmed that the absence of B cells restricted tumour growth in a variety of tumour models. These included TS/A, an implantable mammary adenocarcinoma, J558L, a plasmacytoma, and CMS-5, a fibrosarcoma, all of which were rejected by μMT−/− but not wild-type mice vaccinated with irradiated tumour cells [18]. Increased proliferation of CD4+ T cells and cytotoxic activity of CD8+ T cells from μMT−/− mice was observed in response to immunisation with irradiated TS/A cells in this study, and the effect could be inhibited by adoptive transfer of B cells but not immune serum, suggesting direct effects of T-B collaboration on T cell function [18]. Vaccination with adenoviral vectors expressing melanoma antigens prevented the growth of B16 melanoma in μMT−/− but not wild-type mice and was associated with increase in the magnitude and longevity of the tumour-specific effector T cell response [19]. Shah et al. [20] showed that the growth of MC38 colon carcinoma, EL4 thymoma and B16 melanoma was retarded in unvaccinated μMT−/− mice, and once again the effect was abrogated by adoptive transfer of B cells. Increased granzyme B and IFN-γ expression and increased cytotoxic activity against MC38 targets were observed in T cells from μMT−/− tumour-bearing mice in this study [20]. The inhibitory effect of B cells on the T cell response to MC38 tumour was dependent on B cell expression of OX40L, suggesting a role for T-B collaboration and Th2 differentiation in the suppression of T-dependent anti-tumour effects [21]. Adoptive transfer of wild-type B cells also reversed inhibition of EMT-6 mammary tumour growth in μMT–/– mice [22]. In this case, the effect was associated with recruitment of regulatory T cells (Tregs) and B cells to the tumour and was independent of IL-10 [22]. In contrast, Inoue et al. [23] concluded that IL-10 production by B cells was a major cause of decreased IFN-γ production and increased growth of EL-4 gag thymoma and D5 melanoma, but not MCA304 sarcoma, in wild type compared with μMT−/− mice. Finally, growth of 2 orthotopic squamous cell carcinoma (SCC) cell lines was reduced in JH−/− mice and correlated with increased CD8+ T cell infiltration [24].
In contrast to anti-tumour responses, T cell responses to immunisation with protein antigens are reduced in μMT−/− mice [25–27], and the effect is most pronounced for Th2 differentiation [28]. Reduction in priming to tumour antigens in chronically B cell-depleted animals has been shown for a Friend leukaemia virus-induced leukaemia [29].
Abnormalities in B cell-deficient mice
While the field has gained a greater understanding of T cell and B cell tumour biology using the μMT−/− mice system, it is important to remember that, from a clinical perspective, B cell-deficient mice do not model the situation in patients, whose B cell compartment remains relatively intact prior to and during cancer progression. Mice rendered genetically deficient in B cells manifest several secondary immune abnormalities that may contribute to their tumour-resistant phenotype. Dendritic cell production of the T helper type 1 (Th1) cell-stimulating cytokine IL-12 is increased in μMT−/− mice [30]. In addition to the direct Th1-augmenting effects of IL-12 on T cells, enhanced IL-12 production may decrease B cell germinal centre reactions, reducing antibody affinity and the effect of normal B cell transfer into μMT−/− mice [31]. T cell receptor diversity is also substantially reduced in μMT−/− mice, reflecting the loss of thymic B cells [32]. B cell-deficient mice also have defects in myeloid cell subsets, including specific macrophage populations within the spleen [33].
B cell depletion models (Table 2)
Table 2.
Modification of anti-tumour responses by B cell depletion
| Role of B cells | Model | B cell manipulation | Immune response | References |
|---|---|---|---|---|
| Positive | B16 melanoma | Acute anti-CD20 | Spontaneous | [34] |
| Positive | B16 melanoma | Acute anti-CD20 | Therapeutic inhibition of CD73 | [35] |
| Positive | GL26 glioblastoma | Acute anti-CD20 depletion before tumour challenge | AD-TK + Flt3L vaccination | [36] |
| Positive | FBL leukaemia | Chronic anti-IgM | Transfer of immune T cells | [29] |
| Negative |
AB12 mesothelioma TC1 and LKR lung cancer EL4 thymoma |
Acute anti-CD20 | Spontaneous | [38] |
| Negative | TC1 lung cancer | Acute anti-CD20 | Ad.E7 vaccination | [38] |
Given the immune abnormalities in mouse models with lifelong B cell deficiency, it may be more useful to assess the roles of B cells in anti-tumour immunity using acute B cell depletion. The majority of such models use antibody depletion, often combined with chemotherapy or vaccination, and suggest that B cells make a positive contribution to tumour clearance in a therapeutic setting. In a B16F10 melanoma model, acute B cell depletion using an anti-CD20 mAb before tumour challenge decreased the number of CD4+ and CD8+ T cell effector memory cells and the production of TNF-α and IFN-γ, while subcutaneous tumour burden and lung metastases were increased [34]. A second study in the B16 model used anti-CD20-mediated B cell depletion to demonstrate that tumour regression in response to inhibition of CD73 was at least partly B cell dependent [35]. In the orthotopic GL26 model of glioblastoma in which tumour-protective T cell responses were induced by treatment with an adenovirus vector encoding the cytotoxic molecule herpes simplex virus 1 thymidine kinase (HSV1-TK) plus the cytokine FMS-like tyrosine kinase 3 ligand (Flt3L), overall survival was reduced by anti-CD20 depletion of B cells or anti-CD49d/anti-LFA depletion of marginal zone B cells [36]. Survival of mice that rejected primary tumours was severely affected if B cells were depleted prior to secondary challenge, indicating the importance of B cells for memory responses [36]. In addition, tumour control was also decreased in μMT−/−mice, in contrast to the results described above. It is possible that this difference is due to different effects of B cells in spontaneous anti-tumour responses, compared with those resulting from vaccination. By using Prdmflox/floxCD19Cre/+ mice in which antibody-secreting B cells expressing Blimp-1 are absent, Candolfi et al. elegantly showed that B cell enhancement of the response to vaccination was not dependent on antibody production and was thus likely to reflect antigen presentation by B cells. In a model of Friend murine leukaemia virus-induced leukaemia, chronic B cell depletion via an anti-μ mAb decreased CD4+ and CD8+ T cell priming and cytotoxicity in response to vaccination with irradiated tumour cells or vaccinia virus-encoded antigen [29]. However, survival of tumour-bearing mice after treatment with cyclophosphamide and adoptive transfer of T cells from tumour-vaccinated mice was unaffected by the presence of B cells, suggesting that B cell involvement was limited to the priming phase of the vaccination response. This positive effect of B cells was reproduced in μMT−/− and CD19−/− mice vaccinated with tumour antigen-loaded exosomes [37].
In contrast to the studies described above, acute B cell depletion with an anti-CD20 mAb was reported to improve control in multiple tumour models by Kim et al. [38]. The effect was greater when B cell depletion was combined with vaccination with an adenovirus encoding a tumour antigen.
B cells with regulatory function (Table 3)
Table 3.
Evidence for regulatory B cells
| Role of B cells | Model | B cell manipulation | References |
|---|---|---|---|
| Negative | DMBA-/TPA-induced carcinogenesis | Genetic knockout | [40] |
| Negative | BL3750 lymphoma (CD20+) in WT or CD20−/− hosts | Acute anti-CD20 | [41] |
| Negative | 4T1 breast carcinoma | Genetic knockout | [42] |
| Negative | TRAMP prostate tumour | B cell transfer | [43] |
| Negative |
TRAMP prostate tumour Myc-CaP prostate cancer |
Genetic knockout | [45] |
Over the last decade, researchers have gained a greater understanding of the regulatory roles of B cells in chronic inflammation, autoimmunity and cancer. Regulatory B cell (Breg) function has been extensively reviewed previously [39], and only those aspects relevant to anti-tumour responses will be discussed briefly below. The ability of Breg cells to interfere with protective anti-tumour immune responses has been shown to involve production of IL-10 in a chemical carcinogenesis (7,12-dimethylbenz[a]anthracene/tetradecanoyl phorbol acetate (DMBA/TPA)) model of SCC [40] and in the BL3750 lymphoma model [41]. TGFβ-dependent, IL-10-independent induction of Foxp3 expression in CD4+ T cells was dependent on Bregs in the 4T1 model of murine breast cancer [42].
Secreted products from B cells that do not have a Breg phenotype may also have effects on tumour growth in vivo. In the case of prostate cancer, promotion of castration-resistant progression is at least partly dependent on a specific response of prostate epithelial cells to lymphotoxin derived from B cells [43, 44]. A recent report also implicated TGFβ-dependent B cells (plasmablasts) expressing PD-L1 and secreting IgA and IL-10 in resistance of prostate cancer to oxaliplatin chemotherapy [45]. These studies suggest that many different tumour-promoting mechanisms may be utilised by B cells in tumour models.
Summary of B cell studies
B cell infiltration in human cancer is generally associated with an improvement in prognosis. Many mechanisms have been postulated but little direct functional evidence is available. A negative effect of B cells on spontaneous T cell responses in tumour-bearing animals has been convincingly demonstrated, whereas B cells enhance the anti-tumour response in many murine studies of therapeutic vaccination. Overall, acute B cell depletion models may reflect human disease and therapeutic approaches more accurately than models reliant on lifelong genetic deficiency of B cells.
Antibodies in tumour immunity
Differences in the arrangement of the IgH locus in humans and mice
Human and mouse antibody isotypes differ in both their chromosomal arrangement and function. In the human IgH locus, the constant region genes (in order moving distally from the V, D and J segments) encode IgM, IgD, IgG3, IgG1, IgA1, IgG2, IgG4, IgE and IgA2. The corresponding order in mice is IgM, IgD, IgG3, IgG1, IgG2b, IgG2a, IgE and IgA. Because isotype switching removes all upstream C regions, sequential gamma chain switching in response to persistent antigen in humans will result in production of IgG4, an isotype that has been linked to regulatory effects [46]. In contrast, the most distal murine gamma chain gene is IgG2a, one of the most pro-inflammatory isotypes [47]. This may be an important consideration when comparing chronic anti-tumour responses in mouse and man, along with the much shorter time frame in animal models.
Correlations between serum antibody isotype distribution and patient survival
Human IgG4+ B cells were reported to accumulate in melanoma tissue and their presence correlated with local IL-10 production, but not with a change in patient survival [48]. However, a relative increase in total (not tumour specific) serum IgG4 in patient serum was shown to correlate with decreased survival in this study, while there was a trend towards a positive correlation between serum IgG1 (essentially equivalent to murine IgG2a in its functions) and increased survival [48]. IgG4 was also shown to inhibit IgG1-dependent monocyte killing of tumour cells in vitro [48]. In a second study, an increase in total serum IgG4 was seen in metastatic melanoma compared to primary disease, and high titres were more likely in advanced disease [49].
There is also evidence that IgE may be involved in tumour clearance. In patients with pancreatic cancer, serum IgE was significantly increased and specific antibody-dependent cytotoxicity against pancreatic cancer cells could be demonstrated in vitro [50]. Enhanced survival of patients with glioma has also been associated with increased serum levels of IgE [51]. IgG4 has been shown to inhibit IgE-dependent pro-inflammatory functions mediated via IgE receptor Fc epsilon RI (FcεRI) and IgE receptor Fc epsilon RII (FcεRII) [52], suggesting a possible mechanism for the poor outcomes associated with high serum IgG4.
Effects of human tumour-reactive antibodies
Numerous reports have identified tumour-specific antibodies in the serum of cancer patients [53]. Most anti-tumour antibody detection methods, including serological identification of antigens by recombinant expression cloning (SEREX), were designed to detect switched IgG antibodies, indicative of CD4+ T cell involvement and possibly Treg induction directed against the same tumour antigen [54]. Relatively few studies have attempted to assess the effects of spontaneous tumour-specific antibody responses on patient survival. In a study of pancreatic cancer, the serum titre of antibodies specific for MUC1, a membrane-tethered mucin glycoprotein whose glycosylation is frequently altered in tumour cells, served as a prognostic marker of increased survival, independent of tumour stage [55]. In melanoma, no association between prognosis and antibody to at least one of a large panel of tumour antigens was found, although for a minority of individual tumour antigens, the prognosis was worse for patients with detectable serum antibody, excluding those with metastatic disease [56]. In colorectal carcinoma patients who had undergone complete resection, a correlation between tumour-specific antibody and worse prognosis was reported [57]. In contrast, serum Abs produced by infiltrating B cells could not be detected in ovarian cancer [6]. In advanced melanoma patients treated with the CTLA-4-reactive mAb ipilimumab, antibodies specific for the tumour testis Ag NY-ESO-1 correlated with clinical benefit, although the effect could not be separated from that of enhanced CD8+ T cell involvement [58].
Tumour-reactive antibodies in mouse models (Table 4)
Table 4.
Evidence for tumour antigen-specific antibody effects
| Role of antibody | Model | Antibody isotype | Additional therapy | Reference |
|---|---|---|---|---|
| Positive | B16 melanoma | IgG2a | Nil | [59] |
| Positive | B16 melanoma | IgG2a > IgG2b (not IgG1, IgG3) | Nil | [60] |
| Positive | B16 melanoma | IgG2a | Nil | [61] |
| Positive | B16 melanoma | IgG2a | Anti-VEGF receptor | [62] |
| Positive |
MCA205 fibrosarcoma D5G6 melanoma |
IgG2b | Chemotherapy or irradiation | [63] |
| Positive | HCC-97H human hepatocellular carcinoma | Unspecified | Nil | [64] |
| Positive | B16 melanoma | IgG2a | Peptide vaccine | [65] |
| Positive | RM1 prostate carcinoma (GRP-dependent growth) | Unspecified | GRP-vaccine | [66] |
| Positive | AsPC-1 human pancreatic carcinoma | Human IgG1 | Nil | [67] |
| Positive | Human ovarian carcinoma | IgE (not IgG1) | Transfer of human lymphocytes | [68] |
| Positive | TSA-LACK mammary carcinoma | Human IgE | Vaccination, humanised FcεRIα-transgenic | [69] |
| Positive | CT26 prostate carcinoma expressing human PSA | Human IgE | Vaccination, humanised FcεRIα-transgenic | [70] |
| Negative | K14-HPV16 transgenic SCC | IgG1, IgG2a | Nil | [76] |
| Negative | K14-HPV16 transgenic SCC | IgG1, IgG2a | Nil | [77] |
To pinpoint the precise effects of tumour-specific antibodies, as opposed to tumour-specific B cells, hybridoma technology has been used to generate mAbs for administration in murine tumour models. Early studies using therapeutic administration of TA99, a mouse IgG2a isotype mAb specific for the melanoma Ag gp75, demonstrated positive effects against subcutaneous and metastatic B16F10 tumours [59]. Subsequent studies comparing the effect of antibodies of the same specificity but different isotypes indicated that IgG2a, and to a lesser extent IgG2b, protected mice from lung metastases after intravenous tumour cell inoculation, while IgG1 and IgG3 did not [60]. This phenomenon is due to the intrinsic functions of different Fc receptors (FcR) and has recently been reported to involve neutrophil-mediated cytotoxicity [61]. In addition, mAbs directed to tumour Ags may have different effects in vivo compared to in vitro due to changes in Ag expression. For example, gp75 is poorly expressed on the surface of cultured B16 cells, but is highly expressed in vivo [62].
Tumour-specific antibodies are particularly effective in metastasis models. These include the commonly used B16F10 melanoma model [60], MCA 205 fibrosarcoma and D5 melanoma [63] and a human HCC-97H hepatocellular carcinoma in nude (T cell-deficient) mice [64]. Several laboratories have also reported efficacy of antibodies against subcutaneous tumours. When combined with adjuvanted peptide vaccination, administration of TA99 was able to eradicate a proportion of subcutaneous B16F10 melanomas [65]. A vaccine against gastrin-releasing peptide (GRP) protected against growth of GRP-expressing RM-1 prostate cancer subcutaneous tumours and lung metastases, and immune serum could transfer partial protection [66]. In immunodeficient animal models, administration of purified anti-tumour antibodies was able to inhibit the growth of human pancreatic and colorectal cancer lines [67] and human ovarian cancer xenografts [68]. Recent studies of the anti-tumour effects of human IgE have made use of transgenic mice expressing the human high affinity FcεRI required for IgE isotype-dependent functions [69]. A human IgE specific for prostate-specific antigen (PSA) slowed the growth of CT26 prostate cancer cells expressing human PSA [70].
In many cases, the effects of B cells and antibody in mouse models have not been separated using techniques such as serum transfer or specific inactivation of antibody production. Studies that demonstrated an effect against metastases include that of Sorrentino et al., who showed that growth of B16F10 lung metastases was enhanced in μMT−/− mice, as were concentrations of IL-10 and TGFβ in bronchoalveolar lavage [71]. Adoptively transferred B cells were protective, especially after activation with CpG–ODN [71], and this effect was also seen in T cell-deficient hosts [36]. In the 4T1 breast cancer model, tumour-specific IgG antibody produced after in vitro stimulation of in vivo primed B cells was shown to mediate in vitro complement-dependent tumour cell lysis [72]. In a second mammary carcinoma model, targeting human HER2/neu to B cells via a CD19 single chain variable fragment led to mixed IgG1, IgG2a and IgG2b antibody responses capable of activating complement and inhibiting human breast cancer cell growth in vitro [73]. Administration of the B cell-targeted fusion protein also inhibited the in vivo growth of subcutaneous D2F2/E2 mammary carcinoma cells expressing human HER2/neu [73]. In a second study in the D2F2/E2 model, administration of a human anti-HER2/neu IgE mAb prolonged survival of mice expressing the appropriate human Fc receptor, FcεR1α, after intraperitoneal administration of D2F2/E2 tumour cells [74].
Recently, it has been suggested that the in vivo concentration of tumour-reactive antibodies may significantly affect whether they contribute to tumour progression or clearance [75]. These studies in the MC38 colon carcinoma model showed that polyclonal antisera specific for the Neu5Gc tumour Ag enhanced tumour growth at low concentrations but were inhibitory at higher concentrations. Higher avidity antibodies required lower concentrations for inhibitory effect.
Antibody effects during cancer initiation
In a well characterised mouse model of virally induced SCC, immune complexes containing viral antigen were shown to be the primary drivers of chronic inflammation, which in turn strongly predisposed to malignancy [76]. Malignant transformation was dependent on the presence of both antibody and FcRγ, which contributed directly to chronic skin inflammation [77] and a shift in macrophage phenotype from M2 to the more pro-inflammatory M1 [78]. Interestingly, the predominant isotype deposited in pre-neoplastic skin was mouse IgG1, which is poorly bound by activating FcγRs, and is not generally regarded as a pro-inflammatory isotype. Thus, the lower amounts of highly pro-inflammatory mouse IgG2a may be the major drivers of carcinogenesis in this model. While antibodies have a clear role in progression in this mouse model of SSC, B cell infiltration of tumours was not observed, in contrast to human head and neck SSC where the presence of peritumoural B cells correlated with increased survival [8]. Whether this difference is related to differences in species, oncogenic drivers or the stage of cancer progression remains to be determined.
Summary of antibody studies
Overall these data suggest that tumour-specific antibodies may have diverse roles in anti-tumour immunity. The relative amounts of each antibody isotype and their affinity, the stage of tumour progression and the tumour cell surface Ag expression are all factors that should be considered when evaluating the efficiency of antibody protection in tumour models. Antibody-dependent effects may also be influenced by the number and type of in situ FcR-expressing cells.
It is likely that the isotype and concentration of tumour-specific antibodies reflect the strength and status of the CD4+ T cell response. Antibody may play an atypical role in driving malignant transformation in some cancers, but at the later stages of cancer progression, antibodies appear to have similar effects to those in other chronic immune responses.
Conclusions
At first glance, it appears that B cells play fundamentally different roles in spontaneous anti-tumour immunity in mouse and man. They are generally associated with improved outcomes in clinical disease, and yet often produce worse outcomes in mouse models. Many hypotheses have been advanced to explain this apparent paradox, including differences between human and mouse immune systems, differences between the responses to naturally occurring and transplantable tumours and the artificiality of genetically modified mouse models that affect B cell viability and function.
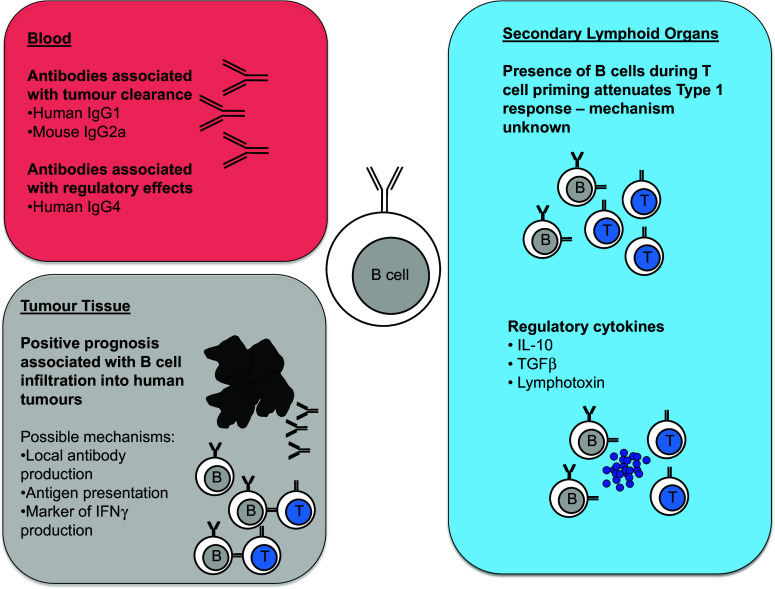
Our view is that the differences between the conclusions drawn from human and mouse studies can be accommodated within a single model if the problem of anti-tumour immunity is considered from the wider perspective of how normal immune responses are regulated (Fig. 1). Despite the current emphasis on distinct types of immune response (Th1, Th2, Th17) and on responses involving only a single arm of the adaptive response (CD4+ vs CD8+ T cells, CD8+ T cells vs B cells), most natural immune responses include a mixture of cell types, cytokines and differentiation states. It is highly unusual to see immune responses that do not simultaneously involve antigen-specific responses by CD4+, CD8+ and B cells (although not all may contribute equally to pathogen clearance, autoimmunity or tumour control). When patients make spontaneous immune responses to their tumour, it is highly likely that all three cell types are involved. Whether or not all cell types are then recruited into primary tumour or metastases is not necessarily a direct reflection of their initial involvement in the anti-tumour response. Indeed, the presence of CD4+, CD8+ and B cells within tertiary lymphoid follicles in tumours such as melanoma is likely to reflect chronicity of response, which is also associated with the presence of ectopic lymphoid tissue in chronic anti-pathogen responses and autoimmunity [79]. Not only is the immune response in most mouse tumour models of much shorter duration, but laboratory studies usually measure the functional immune response in lymph nodes and spleen, rather than documenting infiltration of different immune cells into the tumour.
Fig. 1.
Mechanisms by which B cells affect tumour growth
Mouse models in which B cells are present when T cells are first primed against the tumour usually show detectable immune deviation away from the type 1 response that is highly effective in clearing most tumours. This phenomenon is not restricted to anti-tumour responses, but is a general property of T cell priming in the context of active T-B collaboration in both mouse and man. Published reports have focussed on those examples where this degree of immune deviation has significant effects on tumour growth in mice. However, this does not imply that effective anti-tumour immune responses cannot also be mounted by normal immune systems containing B cells—only that they may have been even more effective had B cells been artificially absent during priming.
In patients who have already made an anti-tumour response at presentation, the question of whether the response would have been stronger if B cells had not been present is not clinically relevant. How preclinical studies of B cells inform therapeutic options at this stage then involves two questions: are B cells currently exerting a positive or negative effect in patients who have already mounted an anti-tumour response and will B cells exert a positive or negative effect in relation to future therapy?
Interestingly, in mouse models in which anti-tumour responses are modulated by vaccination, most studies have shown that B cell depletion has a negative effect, which may be related to enhanced B cell-dependent presentation of vaccine rather than naturally tumour-derived antigen. Acute B cell depletion during chronic anti-tumour responses is also detrimental in most cases. While this appears to run counter to the concept of Breg effects in cancer, B cell depletion removes both positive and negative effects of different B cell subsets. It is not yet clear whether targeted depletion of regulatory but not conventional B cells would be more beneficial and whether this will be possible in the clinic.
The effects of anti-tumour antibody in both mouse and man are dependent on isotype and rely on the same immune components, including FcRs and accessory cells such as neutrophils, monocytes and macrophages. The major species difference is that repeated isotype switching within IgG subclasses in humans generates IgG4, an isotype with regulatory function rather than the pro-inflammatory IgG2a isotype generated in mice. However, the relative importance of this difference in tumour immunity is unclear, particularly as IgG4 is a relatively minor component of human serum IgG.
In summary, there is little evidence to support B cell depletion as an immunoenhancing therapy in human non-B cell cancer. While priming of type 1 immune responses that produce IFN-γ and cytotoxic function is more efficient in the complete absence of B cells, evidence from many different models indicates a positive role for B cells in maintaining and enhancing anti-tumour immunity in both mouse and man.
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia Grants 1012930 and 1051843 and Cancer Council New South Wales Grant RG13-13. Thomas Guy and David Hancock were recipients of Cancer Institute New South Wales Research Scholar awards, and Barbara Fazekas de St Groth was supported by a National Health and Medical Research Council of Australia Principal Research Fellowship.
Abbreviations
- Breg
Regulatory B cell
- CXCR3
CXC chemokine receptor 3
- DMBA
7,12-Dimethylbenz[a]anthracene
- FcR
Fc receptor
- FcεRI
High affinity IgE receptor Fc epsilon RI
- FcεRII
Low affinity IgE receptor Fc epsilon RII
- Flt3L
FMS-like tyrosine kinase 3 ligand
- GRP
Gastrin-releasing peptide
- HSV1-TK
Herpes simplex virus 1 thymidine kinase
- MCA
Methylcholanthrene
- PSA
Prostate-specific antigen
- SCC
Squamous cell carcinoma
- SEREX
Serological identification of antigens by recombinant expression cloning
- Th1
T helper type 1
- TILs
Tumour-infiltrating lymphocytes
- TPA
Tetradecanoyl phorbol acetate
- VCAM-1
Vascular cell adhesion protein 1
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
Footnotes
Elena Shklovskaya, Barbara Fazekas de St Groth are co-authors.
References
- 1.Silina K, Rulle U, Kalnina Z, Line A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother. 2014;63(7):643–662. doi: 10.1007/s00262-014-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serre K, Cunningham AF, Coughlan RE, Lino AC, Rot A, Hub E, Moser K, Manz R, Ferraro A, Bird R, Toellner KM, Demengeot J, MacLennan IC, Mohr E. CD8 T cells induce T-bet-dependent migration toward CXCR3 ligands by differentiated B cells produced during responses to alum-protein vaccines. Blood. 2012;120(23):4552–4559. doi: 10.1182/blood-2012-03-417733. [DOI] [PubMed] [Google Scholar]
- 3.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346(6205):98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladanyi A, Kiss J, Mohos A, Somlai B, Liszkay G, Gilde K, Fejos Z, Gaudi I, Dobos J, Timar J. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60(12):1729–1738. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18(12):3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 7.Shimabukuro-Vornhagen A, Schlosser HA, Gryschok L, Malcher J, Wennhold K, Garcia-Marquez M, Herbold T, Neuhaus LS, Becker HJ, Fiedler A, Scherwitz P, Koslowsky T, Hake R, Stippel DL, Holscher AH, Eidt S, Hallek M, Theurich S, von Bergwelt-Baildon MS. Characterization of tumor-associated B-cell subsets in patients with colorectal cancer. Oncotarget. 2014;5(13):4651–4664. doi: 10.18632/oncotarget.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Herpen CM, van der Voort R, van der Laak JA, Klasen IS, de Graaf AO, van Kempen LC, de Vries IJ, Boer TD, Dolstra H, Torensma R, van Krieken JH, Adema GJ, De Mulder PH. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2008;123(10):2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 9.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72(16):3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 10.Hanker LC, Rody A, Holtrich U, Pusztai L, Ruckhaeberle E, Liedtke C, Ahr A, Heinrich TM, Sänger N, Becker S, Karn T. Prognostic evaluation of the B cell/IL-8 metagene in different intrinsic breast cancer subtypes. Breast Cancer Res Treat. 2013;137(2):407–416. doi: 10.1007/s10549-012-2356-2. [DOI] [PubMed] [Google Scholar]
- 11.Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM, Serody JS. Prognostic B-cell signatures using mRNA-Seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014;20(14):3818–3829. doi: 10.1158/1078-0432.CCR-13-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M, Hellwig B, Hammad S, Othman A, Lohr M, Chen Z, Boehm D, Gebhard S, Petry I, Lebrecht A, Cadenas C, Marchan R, Stewart JD, Solbach C, Holmberg L, Edlund K, Kultima HG, Rody A, Berglund A, Lambe M, Isaksson A, Botling J, Karn T, Muller V, Gerhold-Ay A, Cotarelo C, Sebastian M, Kronenwett R, Bojar H, Lehr HA, Sahin U, Koelbl H, Gehrmann M, Micke P, Rahnenfuhrer J, Hengstler JG. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin kappa C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18(9):2695–2703. doi: 10.1158/1078-0432.CCR-11-2210. [DOI] [PubMed] [Google Scholar]
- 13.Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15(7):1109–1114. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 14.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Jr, Feng L, Sampsel JW. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48(10):541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarella C, Passera R, Magni M, Benedetti F, Rossi A, Gueli A, Patti C, Parvis G, Ciceri F, Gallamini A, Cortelazzo S, Zoli V, Corradini P, Carobbio A, Mule A, Bosa M, Barbui A, Di Nicola M, Sorio M, Caracciolo D, Gianni AM, Rambaldi A. Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol. 2011;29(7):814–824. doi: 10.1200/JCO.2010.28.9777. [DOI] [PubMed] [Google Scholar]
- 16.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. J Immunol. 1978;121(1):359–362. [PubMed] [Google Scholar]
- 17.Brodt P, Gordon J. Natural resistance mechanisms may play a role in protection against chemical carcinogenesis. Cancer Immunol Immunother. 1982;13(2):125–127. doi: 10.1007/BF00205312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4(5):627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 19.Perricone MA, Smith KA, Claussen KA, Plog MS, Hempel DM, Roberts BL, St George JA, Kaplan JM. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27(4):273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, Nechustan H, Challita-Eid PM, Segal BM, Yi KH, Rosenblatt JD. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117(4):574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Morgan R, Podack ER, Rosenblatt J. B cell regulation of anti-tumor immune response. Immunol Res. 2013;57(1–3):115–124. doi: 10.1007/s12026-013-8472-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, Rosenblatt JD. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother. 2013;62(1):87–99. doi: 10.1007/s00262-012-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66(15):7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 24.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, Ma Y, Wiesen JF, Wong MH, Kulesz-Martin M, Irving B, Coussens LM. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25(6):809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165(10):5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 26.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13(12):1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- 27.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 28.Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197(7):875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz KR, Klarnet JP, Gieni RS, HayGlass KT, Greenberg PD. The role of B cells for in vivo T cell responses to a Friend virus-induced leukemia. Science. 1990;249(4971):921–923. doi: 10.1126/science.2118273. [DOI] [PubMed] [Google Scholar]
- 30.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SJ, Caton M, Wang C, Khalil M, Zhou ZJ, Hardin J, Diamond B. Increased IL-12 inhibits B cells’ differentiation to germinal center cells and promotes differentiation to short-lived plasmablasts. J Exp Med. 2008;205(10):2437–2448. doi: 10.1084/jem.20070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172(8):4709–4716. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 33.Crowley MT, Reilly CR, Lo D. Influence of lymphocytes on the presence and organization of dendritic cell subsets in the spleen. J Immunol. 1999;163(9):4894–4900. [PubMed] [Google Scholar]
- 34.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184(7):4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol. 2012;189(5):2226–2233. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candolfi M, Curtin JF, Yagiz K, Assi H, Wibowo MK, Alzadeh GE, Foulad D, Muhammad AK, Salehi S, Keech N, Puntel M, Liu C, Sanderson NR, Kroeger KM, Dunn R, Martins G, Lowenstein PR, Castro MG. B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma. Neoplasia. 2011;13(10):947–960. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013;190(6):2712–2719. doi: 10.4049/jimmunol.1203082. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Fridlender ZG, Dunn R, Kehry MR, Kapoor V, Blouin A, Kaiser LR, Albelda SM. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31(5):446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- 39.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 40.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci USA. 2011;108(26):10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest. 2011;121(11):4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71(10):3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammirante M, Kuraishy AI, Shalapour S, Strasner A, Ramirez-Sanchez C, Zhang W, Shabaik A, Karin M. An IKKalpha-E2F1-BMI1 cascade activated by infiltrating B cells controls prostate regeneration and tumor recurrence. Genes Dev. 2013;27(13):1435–1440. doi: 10.1101/gad.220202.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, Jamieson C, Kane CJ, Klatte T, Birner P, Kenner L, Karin M. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins AM, Jackson KJ. A Temporal model of human IgE and IgG antibody function. Front Immunol. 2013;4:235. doi: 10.3389/fimmu.2013.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4(7):541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 48.Karagiannis P, Gilbert AE, Josephs DH, Ali N, Dodev T, Saul L, Correa I, Roberts L, Beddowes E, Koers A, Hobbs C, Ferreira S, Geh JL, Healy C, Harries M, Acland KM, Blower PJ, Mitchell T, Fear DJ, Spicer JF, Lacy KE, Nestle FO, Karagiannis SN. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest. 2013;123(4):1457–1474. doi: 10.1172/JCI65579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daveau M, Pavie-Fischer J, Rivat L, Rivat C, Ropartz C, Peter HH, Cesarini JP, Kourilsky FM. IgG4 subclass in malignant melanoma. J Natl Cancer Inst. 1977;58(2):189–192. doi: 10.1093/jnci/58.2.189. [DOI] [PubMed] [Google Scholar]
- 50.Fu SL, Pierre J, Smith-Norowitz TA, Hagler M, Bowne W, Pincus MR, Mueller CM, Zenilman ME, Bluth MH. Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clin Exp Immunol. 2008;153(3):401–409. doi: 10.1111/j.1365-2249.2008.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wrensch M, Wiencke JK, Wiemels J, Miike R, Patoka J, Moghadassi M, McMillan A, Kelsey KT, Aldape K, Lamborn KR, Parsa AT, Sison JD, Prados MD. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66(8):4531–4541. doi: 10.1158/0008-5472.CAN-05-4032. [DOI] [PubMed] [Google Scholar]
- 52.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163(5):2944–2952. [PubMed] [Google Scholar]
- 53.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58(10):1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106(3):1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 55.Hamanaka Y, Suehiro Y, Fukui M, Shikichi K, Imai K, Hinoda Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103(1):97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 56.Zornig I, Halama N, Lorenzo Bermejo J, Ziegelmeier C, Dickes E, Migdoll A, Kaiser I, Waterboer T, Pawlita M, Grabe N, Ugurel S, Schadendorf D, Falk C, Eichmuller SB, Jager D. Prognostic significance of spontaneous antibody responses against tumor-associated antigens in malignant melanoma patients. Int J Cancer. 2015;136(1):138–151. doi: 10.1002/ijc.28980. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi T, Takii Y, Maruyama S. Usefulness of serum p53 antibody measurement in colorectal cancer: an examination of 1384 primary colorectal cancer patients. Surg Today. 2014;44(8):1529–1535. doi: 10.1007/s00595-013-0703-5. [DOI] [PubMed] [Google Scholar]
- 58.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995;182(5):1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 61.Albanesi M, Mancardi DA, Jonsson F, Iannascoli B, Fiette L, Di Santo JP, Lowell CA, Bruhns P. Neutrophils mediate antibody-induced antitumor effects in mice. Blood. 2013;122(18):3160–3164. doi: 10.1182/blood-2013-04-497446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel D, Bassi R, Hooper AT, Sun H, Huber J, Hicklin DJ, Kang X. Enhanced suppression of melanoma tumor growth and metastasis by combined therapy with anti-VEGF receptor and anti-TYRP-1/gp75 monoclonal antibodies. Anticancer Res. 2008;28(5A):2679–2686. [PubMed] [Google Scholar]
- 63.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183(5):3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J, Yang JM, Wang HJ, Ru GQ, Fan DM. Synthesized multiple antigenic polypeptide vaccine based on B-cell epitopes of human heparanase could elicit a potent antimetastatic effect on human hepatocellular carcinoma in vivo. PLoS One. 2013;8(1):e52940. doi: 10.1371/journal.pone.0052940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ly LV, Sluijter M, van der Burg SH, Jager MJ, van Hall T. Effective cooperation of monoclonal antibody and peptide vaccine for the treatment of mouse melanoma. J Immunol. 2013;190(1):489–496. doi: 10.4049/jimmunol.1200135. [DOI] [PubMed] [Google Scholar]
- 66.Yong L, Huiyong Z, Jing H, Huaqian W, Xiangbing H, Yanjun M, Xiaoyu G, Li H, Yanan Y, Rongyue C, Hao F, Jingjing L, Jie W. Vaccination with a potent DNA vaccine targeting B-cell epitopes of hGRP induces prophylactic and therapeutic antitumor activity in vivo. Gene Ther. 2010;17(4):459–468. doi: 10.1038/gt.2009.165. [DOI] [PubMed] [Google Scholar]
- 67.Patel SP, Bristol A, Saric O, Wang XP, Dubeykovskiy A, Arlen PM, Morse MA. Anti-tumor activity of a novel monoclonal antibody, NPC-1C, optimized for recognition of tumor antigen MUC5AC variant in preclinical models. Cancer Immunol Immunother. 2013;62(6):1011–1019. doi: 10.1007/s00262-013-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karagiannis SN, Josephs DH, Karagiannis P, Gilbert AE, Saul L, Rudman SM, Dodev T, Koers A, Blower PJ, Corrigan C, Beavil AJ, Spicer JF, Nestle FO, Gould HJ. Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application. Cancer Immunol Immunother. 2012;61(9):1547–1564. doi: 10.1007/s00262-011-1162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nigro EA, Brini AT, Soprana E, Ambrosi A, Dombrowicz D, Siccardi AG, Vangelista L. Antitumor IgE adjuvanticity: key role of Fc epsilon RI. J Immunol. 2009;183(7):4530–4536. doi: 10.4049/jimmunol.0900842. [DOI] [PubMed] [Google Scholar]
- 70.Daniels-Wells TR, Helguera G, Leuchter RK, Quintero R, Kozman M, Rodriguez JA, Ortiz-Sanchez E, Martinez-Maza O, Schultes BC, Nicodemus CF, Penichet ML. A novel IgE antibody targeting the prostate-specific antigen as a potential prostate cancer therapy. BMC Cancer. 2013;13:195. doi: 10.1186/1471-2407-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sorrentino R, Morello S, Forte G, Montinaro A, De Vita G, Luciano A, Palma G, Arra C, Maiolino P, Adcock IM, Pinto A. B cells contribute to the antitumor activity of CpG-oligodeoxynucleotide in a mouse model of metastatic lung carcinoma. Am J Respir Crit Care Med. 2011;183(10):1369–1379. doi: 10.1164/rccm.201010-1738OC. [DOI] [PubMed] [Google Scholar]
- 72.Li Q, Lao X, Pan Q, Ning N, Yet J, Xu Y, Li S, Chang AE. ADOPTIVE transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17:4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma Y, Xiang D, Sun J, Ding C, Liu M, Hu X, Li G, Kloecker G, Zhang HG, Yan J. Targeting of antigens to B lymphocytes via CD19 as a means for tumor vaccine development. J Immunol. 2013;190(11):5588–5599. doi: 10.4049/jimmunol.1203216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daniels TR, Leuchter RK, Quintero R, Helguera G, Rodriguez JA, Martinez-Maza O, Schultes BC, Nicodemus CF, Penichet ML. Targeting HER2/neu with a fully human IgE to harness the allergic reaction against cancer cells. Cancer Immunol Immunother. 2012;61(7):991–1003. doi: 10.1007/s00262-011-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearce OM, Laubli H, Verhagen A, Secrest P, Zhang J, Varki NM, Crocker PR, Bui JD, Varki A. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci USA. 2014;111(16):5998–6003. doi: 10.1073/pnas.1209067111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7(5):411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 77.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17(2):121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang M, Wang Z, Graner MW, Yang L, Liao M, Yang Q, Gou J, Zhu Y, Wu C, Liu H, Zhou B, Chen X. B cell infiltration is associated with the increased IL-17 and IL-22 expression in the lungs of patients with tuberculosis. Cell Immunol. 2011;270(2):217–223. doi: 10.1016/j.cellimm.2011.05.009. [DOI] [PubMed] [Google Scholar]