Abstract
Due to their dual binding capacity, bispecific antibodies (bsAb) can be used to cross-link cytotoxic effector cells with malignant targets and may thereby improve adoptive immunotherapy. In this study, the development and preclinical testing of the quadroma-derived bsAb HD37xT5.16 of the specificity CD19xCD5 is reported. Effector cells used were a population of ex vivo expanded and activated T cells called cytokine-induced killer (CIK) cells expressing CD5. When combined with CIK cells, the cytolytic potency of HD37xT5.16 against CD19 positive B cell lymphoma lines was comparable to that observed with a previously described CD19xCD3 bsAb. Further on, we could demonstrate that bsAb CD19xCD5, in contrast to its CD3-binding counterpart, does not induce proliferation of resting T cells and causes only little activation-induced cell death. Therefore, this novel bsAb binding effector T cells via CD5 may be particularly useful in combination with adoptive transfer of ex vivo activated T cells, e.g., in the setting of adoptive immunotherapy after allogeneic stem cell transplantation. The in vitro studies outlined here support the experimental use of bsAb HD37xT5.16 in preclinical in vivo models for evaluation of its safety and efficacy profile.
Keywords: Bispecific antibody, Cytokine-induced killer cells, Non-Hodgkin’s lymphoma, Adoptive immunotherapy, Targeted immunotherapy
Introduction
Bispecific antibodies (bsAb) are artificial proteins that carry two different antigen-binding sites. By virtue of their dual specificity, bsAb can trigger effector cells via a membrane receptor and at the same time link them to a tumor cell. This interaction may lead to the subsequent killing of the tumor cell [32].
Cytokine-induced killer cells are a heterogeneous population of ex vivo expanded and activated peripheral blood mononuclear cells and have been characterized in great detail [18, 28]. They are generated by the timed addition of IFN-γ, OKT3 and IL-2 for 2–3 weeks. About 90% express the T cell markers CD2, CD3 and CD5, and a variable proportion (10–50%) co-express T and NK cell markers. Both CD3+CD56− and CD3+CD56+ cells contribute to their cytotoxicity. CIK cells develop cytotoxic activity against various lymphoma cells [18, 21, 26, 27] and have been retargeted with bsAb to tumor cells in vivo [24]. They can be easily generated in large amounts [13, 18, 27, 28] and cause MHC-unrestricted cytolysis without prior exposure to target cells. Cytotoxicity is mediated by a perforin/granzyme-dependent mechanism [21, 33]. How CIK cells recognize target cells is not completely understood. Recently, a role for the C-type lectin activating receptor family member NKG2D for targeting myeloma cells has been demonstrated [34]. CIK cells do not elicit toxic effects on normal hematopoietic progenitor cells [29].
To mediate redirected lysis, a bsAb must bind either an already activated effector cell or must activate a resting effector cell by binding to a triggering molecule [30]. Most of the studies investigating bsAb for therapy of malignancies have focused on T lymphocytes as effector cells. For this, T cell activation was achieved by ligation of the T cell receptor-associated CD3 epsilon chain. Such anti-T cell x anti-tumor cell bsAbs have been used for the treatment of non-Hodgkin’s lymphoma and solid tumors like ovarian and renal cell cancer [6, 10, 16, 20]. To become fully activated, T cells require co-stimulatory signals via the CD28 receptor, and lack of co-stimulation may induce anergy.
In this study, cytotoxicity of cytokine-induced killer cells targeted with the newly established HD37xT5.16 bsAb to lymphoma cells was investigated. We also examined apoptosis and proliferation of CIK cells after cross-linking to the target cells. BsAbs using CD5 for T cell binding and redirection may have several advantages, as they may prevent activation-induced cell death and may thus lead to longer survival of CIK cells in vivo. To the best of our knowledge, this is the first description and characterization of a CD19xCD5-reactive bsAb.
Materials and methods
Production and purification of bsAb HD37xT5.16
Bispecific antibody HD37xT5.16 was produced using the mouse hybrid–hybridoma technique. Briefly, bsAb was prepared by fusing the hybridoma cell lines HD37 (IgG1, directed against CD19, the broadest pan B cell antigen known) [22] and T5.16 (IgG2a, directed against CD5 expressed on virtually all mature T cells and a subset of B lymphocytes). After several rounds of subcloning and testing for the secretion of bi-isotypic antibodies, a stable quadroma cell line was established. Quadroma cells were cultured in a Miniperm bioreactor (Greiner, Frickenhausen, Germany). Supernatant containing the antibody mixture was purified first by affinity chromatography on a protein A-Sepharose CL-4B column (Amersham Biotech Europe, Freiburg, Germany) to remove IgG1 parental antibodies. Subsequently, the eluant was subjected to FPLC purification on a Mono Q column (Amersham Biotech Europe) that allowed separation of bispecific antibody using a Tris–HCl/sodium chloride gradient at pH 8.0. Purity of the eluted material was assessed by SDS-PAGE under reducing conditions and was higher than 95%. The recently described bsAb HD37xOKT3 (CD19xCD3) was included in several experiments for comparison [8].
Flow cytometry
Target and effector cells were analyzed for surface markers by indirect immunofluorescence. Approximately 1 × 106 cells were stained with the following mAbs: HD37 (anti-CD19, IgG1), T5.16 (CD5, IgG2a) and HD20 (anti-idiotype, IgG1, serving as a negative control). Isotype-specific, FITC-labeled goat-anti-mouse IgG1 and IgG2a antibodies (Southern Biotechnology Associates, Birmingham, USA) and FITC-conjugated goat anti-mouse IgG/Fc antibodies (Jackson ImmunoResearch, West Grove, USA) were used as second-step reagents. Dead cells were discriminated by propidium iodide staining (Sigma, Deisenhofen, Germany). To prevent mAb interaction with the natural ligand binding site of Fc receptors, all incubations were carried out in the presence of 2.5 mg/ml polyclonal human IgG (Venimmun N, ZLB Behring, Marburg, Germany). Analysis was performed on a FACScan cytometer (Becton Dickinson, Heidelberg, Germany) using CellQuest software.
For apoptosis measurement, CIK cells were labeled after ex vivo expansion with the red membrane dye PKH26 (Sigma). Subsequently, cells were washed with ice cold PBS and stained with Annexin V-FITC (ApoAlert Annexin V Apoptosis Kit, BD Bioscience, Heidelberg, Germany) and analyzed on an FACScan flow cytometer using CellQuest software. A gate was set around the PKH26-positive cell population, representing CIK cells. The number of Annexin V-positive cells within this gate contained apoptotic and dead cells. The results reported are mean values of three independent experiments.
Cell lines
K422 human B cell lymphoma cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The non-Hodgkin’s lymphoma cell line SU-DHL-4, isolated from the peritoneal effusion of a 38-year-old male with diffuse large cell lymphoma was kindly provided by Dr. Ron Levy, Stanford University. Raji B-lymphoma cells (CD19+) and Jurkat T cells (CD5+) were purchased from the American Tissue Type Collection (Manassas, USA). All cell lines were grown in RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% heat-inactivated FCS (C.C. Pro, Oberdorla, Germany) and 2 mM L-glutamine (Biochrom).
Generation of CIK cells
Peripheral blood mononuclear cells (PBMNCs) were isolated from buffy coat obtained from healthy donors via the blood bank of the University of Heidelberg. Peripheral blood mononuclear cells (PBMNCs) were isolated by ficoll density gradient centrifugation, washed and resuspended 2 × 106 cells/ml in complete RPMI containing 10% fetal calf serum (C.C. Pro), penicillin 100 U/ml, streptomycin 100 U/ml and 2 mmol/l L-glutamine. On day 1, IFN-γ (Imukine, Boehringer-Ingelheim, Ingelheim, Germany) was added to the final concentration of 1,000 U/ml. On day 2, cells were transferred into cell culture flasks coated with antibody to CD3 (Orthoclone-OKT3, Janssen-Cilag, Neuss, Germany) at a final concentration at 25 ng/ml. At the same time, IL-2 (Aldesleukin, Proleukin, Chiron, Ratingen, Germany) at a final concentration of 300 U/ml was added. Cultures were fed every 3 to 4 days with complete RPMI medium and IL-2 (300 U/ml) for a total of 14 days (termed below as Culture 1) or 21 days (Culture 2).
Cytotoxicity assay
In this assay, target cells were labeled with chromium-51 and incubated with CIK cells. The amount of 51Cr released into the supernatant correlates with cell lysis and is a measure of CIK-cell-mediated cytotoxicity. Below, a standard assay at an effector to target ratio of 10:1 is described: Target cells were harvested, washed twice and resuspended in complete RPMI; 1 × 106 cells in complete medium were labeled with 200 μCi Na2-51CrO4 for 1 h at 37°C. Effector cells were washed and resuspended to 2 × 105 cells/ml. Then, 100 μl of cells were pipetted out into 96 U-bottomed plates in triplicate. Labeled target cells were washed three times and resuspended at a concentration of 2 × 104 cells/ml in complete medium. Next, 100 μl of cells were then added into 96 U-bottomed plates in triplicates to give a total volume of 200 μl. Then, 10 μl of bispecific antibodies with a final concentration of 1 μg/ml was added and incubated for 4 h at 37°C and 5% CO2. Spontaneous release was obtained by incubating target cells in complete medium alone and maximal release was determined by incubating 100 μl 1% Triton X-100 (Merck, Darmstadt, Germany) with 100 μl target. After the 4 h incubation time, the supernatant was carefully removed and counted in a gamma counter (Cobra Auto Gamma, PerkinElmer Life and Analytical Sciences, Rodgau-Jügesheim, Germany). The cytotoxicity was determined using the mean cpm for each triplicate of wells and calculated according to the formula,
 |
The results reported are mean values of three independent experiments done in triplicate.
Proliferation assay
Proliferation of cells was measured by incorporation of [3H]-thymidine. Maxisorp microtitre plate were coated with the appropriate bispecific antibody and incubated overnight at 4°C. Freshly prepared PBMNCs from healthy donors and CIK cells were cultured in round-bottom 96 well plates with or without bsAb at a total of 105 cells/well for 1–3 days in complete medium in a final volume of 200 μl/well at 37°C and 7% CO2. At 18 h before the end of the assay, 1 μCi of [3H]-thymidine was added. The cells were harvested and the incorporated radioactivity was determined by liquid scintillation counting (Wallac 1414 Liquid Scintillation Counter, PerkinElmer Life and Analytical Sciences) and given as cpm. All assays were done with a bsAb end concentration of 1 μg/ml. The results reported are mean values of three independent experiments.
Statistical analysis
Specific lysis, apoptosis and thymidine incorporation in the cell populations analyzed were evaluated using the paired t test.
Results
Establishment of the quadroma line, production and purification of bsAb HD37xT5.16
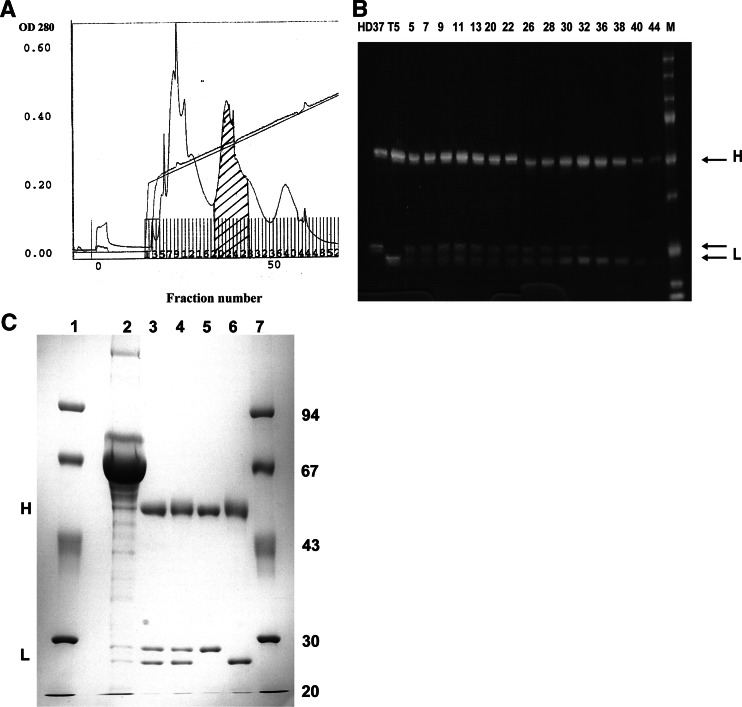
A quadroma cell line was raised by fusion of the HGPRT-deficient hybridoma T5.16 (anti-CD5, IgG2a isotype) and the iodoacetamide-treated hybridoma HD37 (anti-CD19, IgG1 isotype). A total of 45 clones grew out under selection in HAT medium. They were initially tested for the production of both parental immunoglobulin species by a double isotype ELISA and for reactivity with cells expressing the respective antigens using flow cytometry. The quadroma line showing the highest immunoglobulin secretion rate was subsequently cloned twice by limiting dilution and established as hybrid-hybridoma clone HD37xT5.16. For medium-scale production of bsAb, a modular miniaturized bioreactor was employed. BsAb was purified by protein A affinity chromatography and subsequent FPLC separation on a Mono Q column. The FPLC elution profile using a sodium chloride gradient in Tris–HCl is depicted in Fig. 1a.
Fig. 1.
a Purification of bsAb HD37xT5.16 by FPLC separation on a Mono Q column. Elution was performed using a sodium chloride gradient in Tris–HCl (pH 8.0). The hatched peak contained the bispecific antibody. b SDS-PAGE analysis under reducing conditions of HD37xT5 16 preparations in comparison with parental monoclonal antibody. Lanes HD37 and T5, parental mAbs; lane M, molecular weight marker; lanes 5–44, FPLC-eluted fractions. Fractions 20 to 27 contained the bsAb and were pooled. c SDS-PAGE analysis under reducing conditions of HD37xT5.16 bsAb before and after purification. Lanes 1 and 7, molecular weight marker; lane 2, quadroma supernatant; lane 3, protein A-Sepharose CL-4B purified material; lane 4, FPLC-purified bsAb pool; lane 5, parental mAb HD37; lane 6, parental mAb T5.16. The positions of immunoglobulin heavy (H) and light (L) chains are indicated
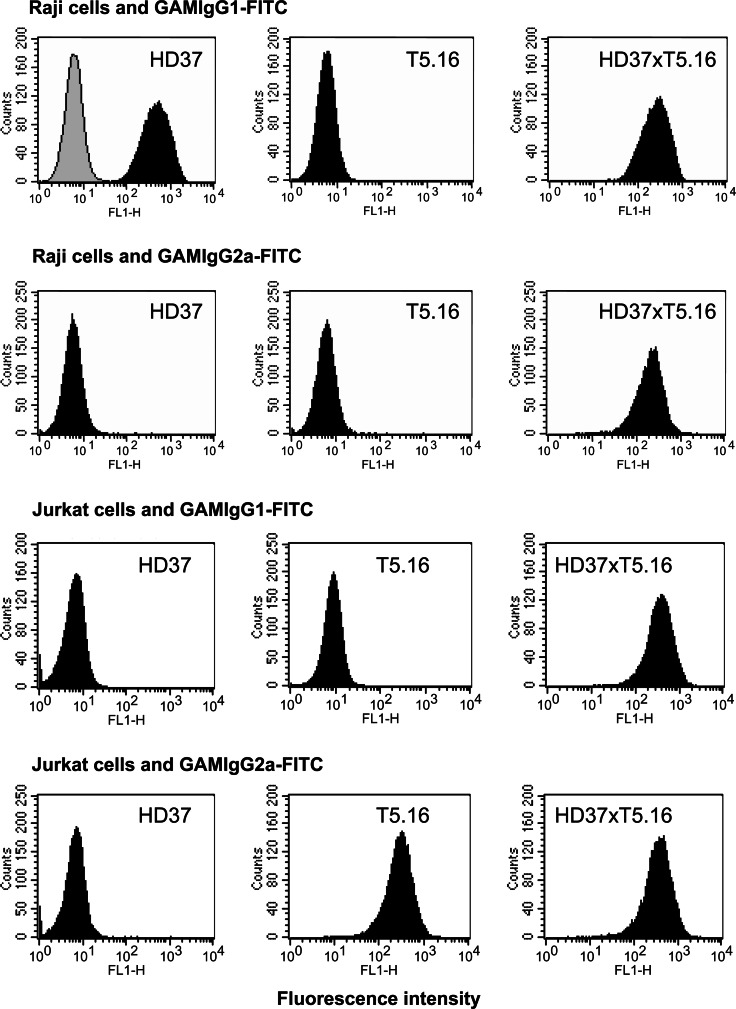
With SDS-PAGE, the apparent electrophoretic mobilities of the parental antibodies differed in the case of the light chains. This allows a rough estimate of the relative amounts of both parental immunoglobulins. After protein A purification of the crude bioreactor-derived supernatant (fractions 5–44), a clear overrepresentation of IgG1 immunoglobulin species containing bsAb and parental antibody HD37 was seen, which also reflects a loss of parental antibody T5.16. By a further purification procedure using a Mono Q column, the parental HD37 antibody was removed, resulting in a high enrichment of bsAb (fractions 20–27), as indicated by the nearly equimolar representation of both immunoglobulin light chains. A representative SDS-PAGE under reducing conditions is displayed in Fig. 1b. The yield of bsAb HD37xT5.16 was in the range of 10–15% of total immunoglobulin. To assess the purity of the pooled bsAb fractions, a separate gel with high resolution was run comparing quadroma supernatant, initial protein A-purified material and the bsAb pool after FPLC separation that was used in all further experiments (Fig. 1c). Essentially, no contaminants were detectable in the final bsAb preparation, indicative of purity higher than 95%. Functional activity of bsAb was evaluated by flow cytometric detection of labeled antibody binding to CD5 on Jurkat or CD19 on Raji cells (Fig. 2). The parental mAb T5.16 (IgG2a) binds to its antigen on Jurkat cells and can solely be detected with FITC-labeled GAM-IgG2a antibodies. Since CD19 antigen is not expressed on Jurkat cells, no binding was observed. By contrast, bsAb HD37xT5.16 binding to CD5 antigen was detected by both isotype-specific antibodies GAM-IgG1- and GAM-IgG2a-FITC. The corresponding staining was performed on Raji cells. To minimize nonspecific Fc receptor-mediated binding, all incubations were carried out in a medium containing 5% pooled human IgG. On Raji cells, parental antibody HD37 (IgG1) bound to the CD19 antigen and was detected by GAM-IGg1-FITC second step reagent; mAb T5.16 did not show any specific reaction with Raji cells that are devoid of the CD5 antigen. Again, only bsAb HD37xT5.16 was reactive with Raji cells using both GAM-IgG1- and GAM-IgG2a-FITC secondary antibodies. These results clearly demonstrated that the two binding sites of the bispecific antibody are functional in that they interact with both, CD5 and CD19 molecules.
Fig. 2.
Flow cytometric analysis of reactivity of bsAb HD37xT5.16. Binding of indicated monoclonal antibody and bispecific antibody to Jurkat (CD5+ and CD19−) and Raji (CD5− and CD19+) cells was detected with isotype-specific, FITC-labeled second-step reagents. The gray histogram represents a negative control with irrelevant monoclonal antibody HD20
CIK cells efficiently kill B-cellular lymphoma targets in the presence of bsAb HD37xT5.16
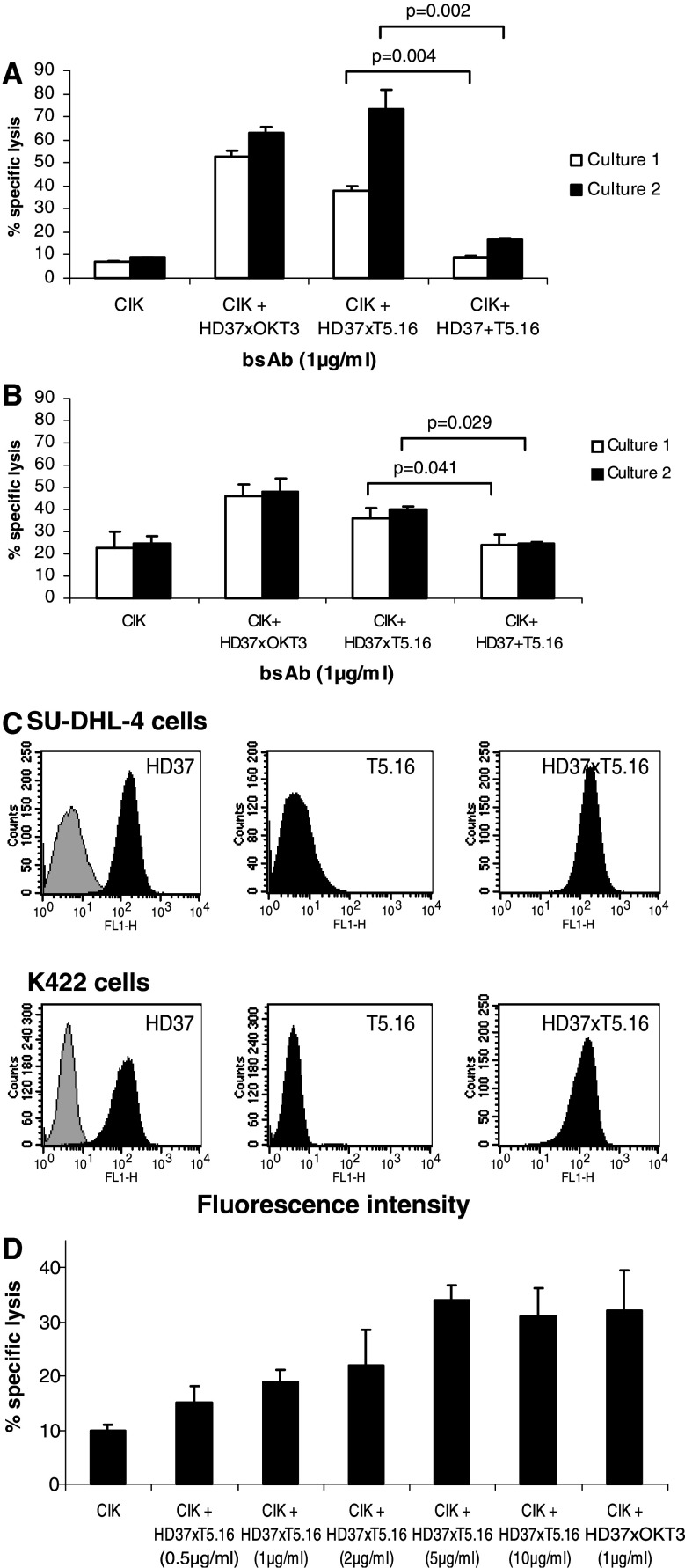
The ability of bsAb HD37xT5.16 to direct CIK cells against lymphoma cells was tested in a cytotoxicity assay. For this, CIK cells from healthy donors were generated and cultured as stated in “Materials and methods” and two different B cell lines were employed as targets. In the presence of 1 μg/ml bsAb HD37xT5.16, a standard chromium release assay was carried out. The parental antibodies HD37 and T5.16, alone and as a mixture, at the same concentration as HD37xT5.16 as well as medium, served as negative controls. The results of the cytotoxicity assay are shown in Fig. 3. At an E:T cell ratio of 10:1, bsAb HD37xT5.16 yielded a specific lysis of 38 and 72% for CIK culture 1 and 2, respectively, with cell line K422 (Fig. 3a). A specific lysis of 37 and 40% was observed for CIK culture 1 and 2, respectively with cell line SU-DHL-4 (Fig. 3b). Addition of parental mAbs or a mixture of them resulted in a background lysis of 15% or less with K422 cells and a background lysis of 22% with SU-DHL-4. Differences between parental antibodies and bsAb were statistically significant in all combinations of cell lines and CIK cultures tested (P < 0.05). The cytotoxicity induced by bsAb HD37xT5.16 was comparable to that obtained with bsAb HD37xOKT3, which was included in the experiment as a positive control. Target cell lines, SU-DHL-4 and K422, expressed intermediate amounts of CD19 and were devoid of CD5 as revealed by flow cytometry. Interestingly, bsAb HD37xT5.16 showed slightly higher mean fluorescence intensity than the parental HD37 mAb when probed with a second-step reagent reactive with all mouse IgG isotypes (MFI 119 of HD37 vs. 131 of HD37xT5.16 in case of SU-DHL-4 cells, and MFI 156 of HD37 vs. 181 of HD37xT5.16 in case of K422 cells; Fig. 3c).
Fig. 3.
BsAb HD37xT5.16 elicits cytotoxic activity of CIK cells towards B-lymphoma cell lines. a BsAb HD37xT5.16 induced cytotoxicity against lymphoma cell line K422. b BsAb HD37xT5.16 induced cytotoxicity against lymphoma cell line SU-DHL-4. Indicated antibodies were added at a final concentration of 1 μg/ml and 51Cr-labeled target cell were incubated with effector cells at an E:T ratio of 10:1 for 4 h. CIK cells from healthy donor after 2 weeks (culture 1) and 3 weeks (culture 2) were employed. c Phenotype of target cell lines SU-DHL-4 and K422 with regard to CD19 (HD37), CD5 (T5.16) and bsAb (HD37xT5.16) expression. The gray histograms represent a negative control by non-binding mAb HD20. d Dose–response curve of bsAb HD37xT5.16 at increasing concentrations compared with HD37xOKT3 (1 μg/ml) employing CIK cells cultured for 2 weeks and SU-DHL4 cell line. Data from three different independent experiments done in triplicate are presented with standard deviation
A dose–response relationship for bsAb HD37xT5.16 was established using CIK cells from healthy donor and SU-DHL-4 target B cells (Fig. 3d). Specific target cell lysis started at 0.5 μg/ml and reached a plateau at bsAb concentration of 5 μg/ml. With this concentration, lysis was comparable to that achieved with the previously described bsAb HD37xOKT3 at 1 μg/ml.
BsAb HD37xT5.16 does not induce proliferation of resting T cells from peripheral blood
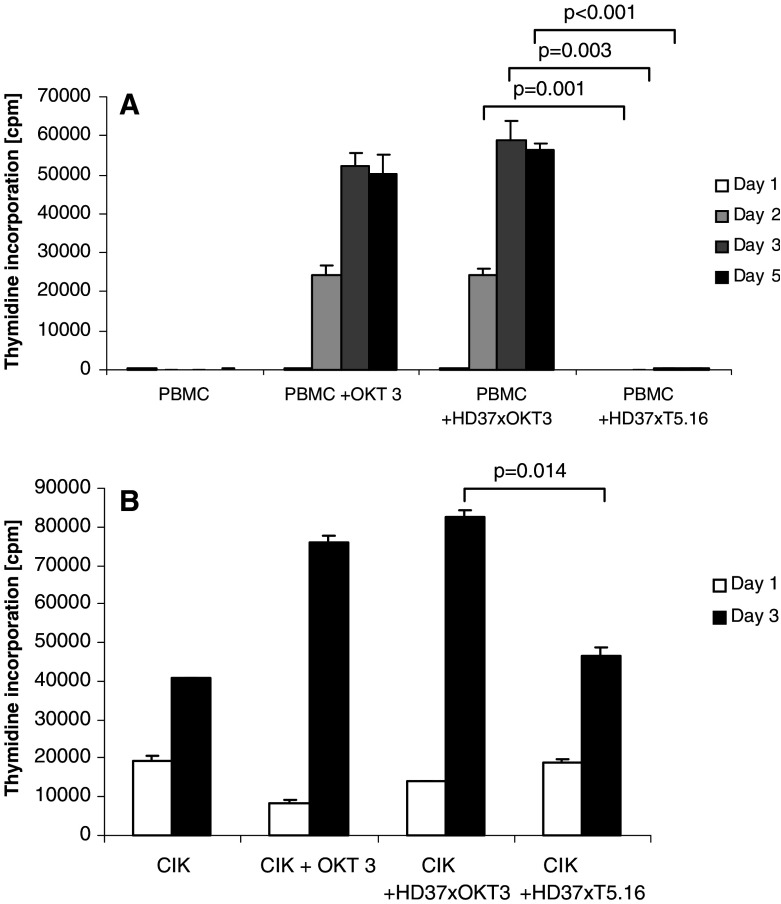
In order to analyze the capacity of the bsAb HD37xT5.16 to activate resting T cells, proliferation of cells was measured by incorporation of [3H]-thymidine. Cells were harvested and radioactivity incorporated into DNA was determined by liquid scintillation counting. Figure 4a shows proliferative activity of two immobilized bsAb, CD19xCD3 and CD19xCD5, on naive T cells. Expectedly, a high proliferation rate was observed in naive T cells with CD3-binding bsAb HD37xOKT3 on days 3 and 5. Essentially the same effect was seen when mAb OKT3 was bound to the culture plates. In sharp contrast, bsAb HD37xT5.16 did not induce T-cell proliferation at all (P < 0.01). These results clearly demonstrate that incubation of naive T cells with bsAb HD37xT5.16, and the consequential cross-linking of CD5 complexes, does not deliver a signal sufficient for the induction of T-cell proliferation. Figure 4b shows proliferation of pre-activated T cells (CIK cells) with and without immobilized bsAb. Here, HD37xOKT3 increased proliferation of CIK cells (82,000 cpm) as OKT3 bivalent antibody did (76,000 cpm), while the proliferation observed with HD37xT5.16 was comparable to CIK cells alone (45,000 and 42,000 cpm, respectively) on the 3rd day of incubation (P < 0.05).
Fig. 4.
Comparison of proliferative response in PBMNCs and CIK cells triggered by immobilized bsAb HD37xT5.16 and HD37xOKT3. a Proliferation of PBMNCs with and without immobilized bsAb and control mAb OKT3. b Proliferation of CIK cells with and without immobilized bsAb and control mAb OKT3. Indicated antibodies were coated on a Maxisorb microtiter plate at a final concentration of 1 μg/ml. Data from three experiments are presented with standard deviation
Apoptosis induced in CIK cells during bsAb-mediated interaction with target cells
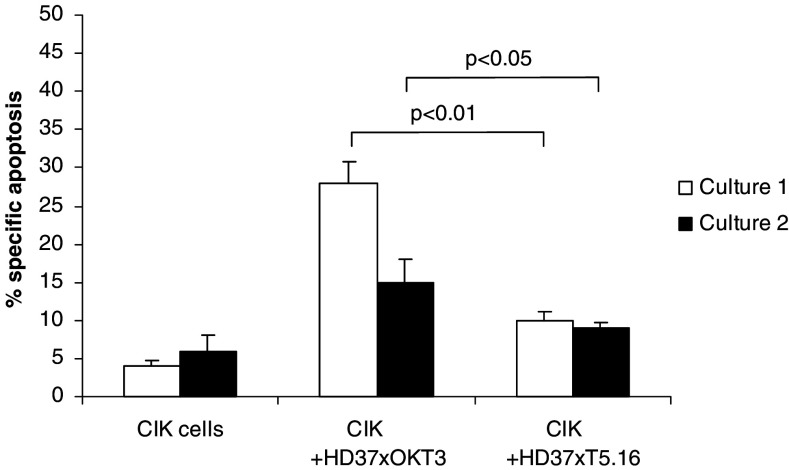
To determine whether effector cells undergo apoptosis when redirected to tumor targets by bsAb, a flow cytometry-based assay was used. Effector cells (CIK cells) were first incubated with the membrane dye PKH26, which allows them to be distinguished from target cells by flow cytometry. For analysis of apoptosis, Annexin V-FITC binding to sphingomyelin residues exposed on the outer leaflet of the cell membrane during early apoptosis and propidium iodide staining of dead cells were used. Using this methodology, the induction of apoptosis in CIK cells after 4 h of incubation with tumor targets and bsAb under various conditions was analyzed. CIK cells incubated with target cells alone or CIK cells alone served as controls. Figure 5 shows apoptosis induced in effector (CIK) cells when incubated with and without bsAb with K422 lymphoma cell line as target. Specific apoptosis of 10 and 9% (CIK culture 1 and 2, respectively) occurred when using bsAb HD37xT5.16, compared to 28 (P < 0.01) and 15% (P < 0.05) with bsAb HD37xOKT3 and less than 6% specific apoptosis without cross-linking antibodies (control). Similar results were obtained with SU-DHL-4 lymphoma cell line. No significant apoptosis was observed with any of the bsAb when used with naive T cells (result not shown).
Fig. 5.
Apoptosis induction in CIK cells following interaction with target cells (K422 cell line) and bispecific antibody. CIK cells from healthy donor after 2 weeks (culture 1) and 3 weeks (culture 2) were employed. The indicated antibodies were added at a final concentration of 1 μg/ml. Data from three experiments are presented with standard deviations
Discussion
The use of bispecific antibodies is aimed at increasing effector cell targeting and enhancing anti-tumor specificity. To mediate redirected lysis, a bsAb must either bind to an already activated effector cell or must activate a resting effector cell by binding to a triggering molecule [30]. In the present study, cytotoxicity of cytokine-induced killer cells towards B-lymphoma cells targeted with the newly established HD37xT5.16 bsAb was investigated. This antibody connects CD5 on effector T cells with CD19 on target cells. We also examined apoptosis induction and proliferation of CIK cells during interaction with the target cells. BsAb increased specific lysis and specific apoptosis in lymphoma cells significantly. This demonstrates that cross-linking CIK cells with target cells by a bsAb can overcome the need for target cell activation and cell adhesion for effector function.
The quadroma line HD37xT5.16 (CD19xCD5) was generated by cell fusion of the parental hybridoma lines. Larger quantities of bsAb were produced in a miniaturized bioreactor and were subsequently purified by a two-step method taking advantage of protein-A CL-4B affinity chromatography that removed the parental T5.16, followed by FPLC separation on a Mono Q column that separated the desired bsAb from mismatched combinations. The yield of bsAb HD37xT5.16, isolated from the hybrid-hybridoma supernatant, was between 10 and 15% of total immunoglobulin content. Flow cytometric analysis revealed the desired functional activity of purified bsAb.
In vitro experiments further demonstrated that bsAb HD37xT5.16 was able to effectively recruit CIK cells for killing CD19 positive lymphoma cell lines. SU-DHL-4 cells are well established as experimental targets for CIK cells [28]. We have elected to also include cell line K422 in our studies, as this cell line is not targeted efficiently by CIK cells without bsAb-mediated cross-linking. Interestingly, with bsAb, cytotoxicity of CIK cells is similar against both cell lines, suggesting that retargeting of CIK cells with HD37xT5.16 may be effective in lymphoma cells resistant to spontaneous CIK cell cytotoxicity.
Specific lysis of target cells in a standard chromium release assay with CIK cells cross-linked via CD5 using bsAb HD37xT5.16 at an effector to target ratio of 10:1 reached 38–70% specific lysis. Comparable results have been achieved with bsAb HD37xOKT3. The latter reagent was included in the experiments because CD3-targeting antibodies have been extensively tested before [2–4, 7, 8, 10, 12, 14, 19, 25]. The resulting activation of effectors is thought to be one of the key events explaining anti-tumor activity of T cells binding to their target via bsAbs. Contrasting with this view, using pre-activated CIK cells as effectors, we found a similar cytotoxicity with the CD5-binding bsAb HD37xT5.16 as compared to the CD3-binding bsAb. In this case, the factor responsible for increased cytolysis after bsAb-mediated cross-linking may be primarily the close proximity between effector and target cells. This may stimulate cytotoxic effectors through ligand–receptor interaction of molecules involved in cell adhesion and non-MHC-restricted immunity [15]. Differences in specific lysis of individual CIK cell cultures are most likely due to variations in T-cell function and activation status between the different healthy blood donors used for the generation of CIK cells. With regard to the potential use of CIK cells in the clinical setting, we did not observe a functional difference in CIK cells expanded from healthy blood donors or from patients suffering from lymphoma or breast cancer, and expression of surface markers was not different between tumor patients and healthy controls (unpublished results). Also, CIK cells generated from tumor patients were analyzed in vitro [15] and used in a clinical phase I trial [17].
Quadroma-derived mouse bispecific antibodies composed of IgG1 and IgG2a immunoglobulin heavy chains (like HD37xOKT3, HD37xT5.16 and others) are poor mediators of antibody-dependent cytotoxicity (ADCC) in human effector cells due to their weak interaction with activating Fc-gamma receptors as CD16 and CD64. To improve binding to Fc-receptors, so-called trifunctional bispecific antibodies combining mouse IgG2a and rat IgG2b immunoglobulins have been engineered. These reagents are able to efficiently recruit and activate accessory cells as macrophages, natural killer cells and dendritic cells leading to T-lymphocyte- and NK cell-mediated target cell lysis and cytokine release [23, 36]. A role of ADCC for retargeting CIK cells by bsAb HD37xT5.16 seems unlikely since the majority of CIK cells represent T lymphocytes and only approximately 10% do express CD16 [27]. To further exclude a contribution of ADCC, we have tried to retarget CIK cells with therapeutic antibodies against CD20 (Rituximab, chimeric human IgG1) and CD52 (Alemtuzumab, humanized IgG1), known to induce ADCC of human effector cells. However, the cytotoxicity towards CD20- or CD52-expressing B-lymphoma target cells was not improved (unpublished data).
Understanding the fate of T cells following cross-linking to tumor targets may be of help in developing effective protocols for clinical trials. Therefore, the proliferation and apoptosis of T-cell effectors after interaction with bsAb was analyzed. For this, we investigated proliferation by immobilizing antibodies onto the plastic surface of cell culture vessels, allowing for the cross-linking of antigens on the surface of effector cells. No significant proliferation was observed when naive T cells were incubated with bsAb HD37xT5.16. These data demonstrate that CD5-binding bsAbs, which are not able to cross-link the TCR, do not provide a signal for proliferation of resting T cells. As shown in Fig. 4a, b, bsAb HD37xOKT3, reacting with TCR-associated CD3 epsilon-chain, readily activates and induces proliferation in naive T cells and CIK cells. The results are not surprising as bsAbs with specificity for CD3 and CD19 have been used for the activation and retargeting of T cells against lymphoma and leukemia cells in vitro and in vivo [3, 8, 10, 12, 25]. The inability of CD5-directed bsAb to activate T cells may reduce its efficacy when used in combination with resting T cells, but this is not relevant when bsAbs are used for retargeting CIK cells that have been pre-activated ex vivo. Indeed, a better killing was not observed with this bsAb in our experiments when compared to bsAb HD37xT5.16. More importantly, CD5 targeting bsAb may be advantageous when applied after allogeneic stem cell transplantation, because the unwanted non-specific activation of T cells that may be directed against antigens of the allogeneic host would not occur. Therefore, the danger of inducing graft versus host disease in the recipient might be reduced.
CIK cells represent activated T cells, and as such they undergo activation-induced cell death (AICD) upon re-ligation of the TCR [1, 5, 11, 31]. AICD is in part mediated through auto and paracrine interactions between Fas and FasL that are both expressed on CIK cells [35]. Cellular proliferation and apoptosis induction are tightly linked processes. Moreover, it has previously been shown that upon tumor encounter, some activated T cells proliferate and then undergo apoptosis [9]. As expected, bsAb HD37xT5.16 targeting CIK cells via CD5 induced less apoptosis than CD3-binding bsAb HD37xOKT3. Thus, targeting of T cells through HD37xT5.16 may have the advantage of preventing AICD and may thus lead to longer survival of CIK cells in vivo.
In conclusion, bsAb HD37xT5.16 increases cytolytic activity of CIK cells against SU-DHL4 and K422 lymphoma cells. This increase in cytotoxicity is similar to that observed with bsAb HD37xOKT3. BsAb recruiting effector cells via CD5 may be advantageous when used with CIK cells, since induction of AICD is drastically reduced. CD5 targeting bsAbs may also offer a benefit in the setting of allogeneic stem cell transplantation, as these bsAb neither activate nor induce proliferation of naive T cells potentially directed against host antigens. The preclinical studies described here support the experimental use of bsAb HD37xT5.16 for adoptive immunotherapy with activated effector T cells.
Acknowledgments
We thank Dr. Fabrice Le Gall (Affimed Therapeutics, Heidelberg) for the advice and help with the FPLC separation and Elvira Hallauer for expert technical assistance. This work was supported by the Deutsche Krebshilfe and the Tumorzentrum Heidelberg/Mannheim.
Footnotes
Freddy Tita-Nwa and Gerhard Moldenhauer contributed equally to the study and share first authorship.
References
- 1.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle PA, Kufer P, Lutterbuse R. Bispecific antibodies for polyclonal T-cell engagement. Curr Opin Mol Ther. 2003;5:413–419. [PubMed] [Google Scholar]
- 3.Bohlen H, Hopff T, Manzke O, Engert A, Kube D, Wickramanayake PD, Diehl V, Tesch H. Lysis of malignant B cells from patients with B-chronic lymphocytic leukemia by autologous T cells activated with CD3 x CD19 bispecific antibodies in combination with bivalent CD28 antibodies. Blood. 1993;82:1803–1812. [PubMed] [Google Scholar]
- 4.Bohlen H, Manzke O, Patel B, Moldenhauer G, Dorken B, von Fliedner V, Diehl V, Tesch H. Cytolysis of leukemic B-cells by T-cells activated via two bispecific antibodies. Cancer Res. 1993;53:4310–4314. [PubMed] [Google Scholar]
- 5.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell–autonomous Fas (CD95)/Fas–ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 6.Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, Eggermont AM, Goey SH, Gratama JW, Lamers CH, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst. 1995;87:1463–1469. doi: 10.1093/jnci/87.19.1463. [DOI] [PubMed] [Google Scholar]
- 7.Cochlovius B, Kipriyanov SM, Stassar MJ, Christ O, Schuhmacher J, Strauss G, Moldenhauer G, Little M. Treatment of human B cell lymphoma xenografts with a CD3 x CD19 diabody and T cells. J Immunol. 2000;165:888–895. doi: 10.4049/jimmunol.165.2.888. [DOI] [PubMed] [Google Scholar]
- 8.Csoka M, Strauss G, Debatin KM, Moldenhauer G. Activation of T cell cytotoxicity against autologous common acute lymphoblastic leukemia (cALL) blasts by CD3xCD19 bispecific antibody. Leukemia. 1996;10:1765–1772. [PubMed] [Google Scholar]
- 9.Daniel PT, Kroidl A, Cayeux S, Bargou R, Blankenstein T, Dorken B. Costimulatory signals through B7.1/CD28 prevent T cell apoptosis during target cell lysis. J Immunol. 1997;159:3808–3815. [PubMed] [Google Scholar]
- 10.de Gast GC, Haagen IA, van Houten AA, Klein SC, Duits AJ, de Weger RA, Vroom TM, Clark MR, Phillips J, van Dijk AJ, et al. CD8 T cell activation after intravenous administration of CD3 x CD19 bispecific antibody in patients with non-Hodgkin lymphoma. Cancer Immunol Immunother. 1995;40:390–396. doi: 10.1007/BF01525390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 12.Haagen IA, de Lau WB, Bast BJ, Geerars AJ, Clark MR, de Gast BC. Unprimed CD4+ and CD8+ T cells can be rapidly activated by a CD3 x CD19 bispecific antibody to proliferate and become cytotoxic. Cancer Immunol Immunother. 1994;39:391–396. doi: 10.1007/BF01534426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyle C, Bangs CD, Chang P, Kamel O, Mehta B, Negrin RS. Expansion of Philadelphia chromosome-negative CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92:3318–3327. [PubMed] [Google Scholar]
- 14.Kipriyanov SM, Moldenhauer G, Strauss G, Little M. Bispecific CD3 x CD19 diabody for T cell-mediated lysis of malignant human B cells. Int J Cancer. 1998;77:763–772. doi: 10.1002/(SICI)1097-0215(19980831)77:5<763::AID-IJC16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Kornacker M, Moldenhauer G, Herbst M, Weilguni E, Tita-Nwa F, Harter C, Hensel M, Ho AD. Cytokine-induced killer cells against autologous CLL: direct cytotoxic effects and induction of immune accessory molecules by interferon-gamma. Int J Cancer. 2006;119:1377–1382. doi: 10.1002/ijc.21994. [DOI] [PubMed] [Google Scholar]
- 16.Kroesen BJ, Bakker A, van Lier RA, The HT, de Leij L. Bispecific antibody-mediated target cell-specific costimulation of resting T cells via CD5 and CD28. Cancer Res. 1995;55:4409–4415. [PubMed] [Google Scholar]
- 17.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 19.Manzke O, Titzer S, Tesch H, Diehl V, Bohlen H. CD3 x CD19 bispecific antibodies and CD28 costimulation for locoregional treatment of low-malignancy non-Hodgkin’s lymphoma. Cancer Immunol Immunother. 1997;45:198–202. doi: 10.1007/s002620050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marme A, Strauss G, Bastert G, Grischke EM, Moldenhauer G. Intraperitoneal bispecific antibody (HEA125xOKT3) therapy inhibits malignant ascites production in advanced ovarian carcinoma. Int J Cancer. 2002;101:183–189. doi: 10.1002/ijc.10562. [DOI] [PubMed] [Google Scholar]
- 21.Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood. 1995;86:3493–3499. [PubMed] [Google Scholar]
- 22.Pezzutto A, Dorken B, Rabinovitch PS, Ledbetter JA, Moldenhauer G, Clark EA. CD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferation. J Immunol. 1987;138:2793–2799. [PubMed] [Google Scholar]
- 23.Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–2534. doi: 10.1182/blood.V98.8.2526. [DOI] [PubMed] [Google Scholar]
- 24.Scheffold C, Kornacker M, Scheffold YC, Contag CH, Negrin RS. Visualization of effective tumor targeting by CD8+ natural killer T cells redirected with bispecific antibody F(ab’)(2)HER2xCD3. Cancer Res. 2002;62:5785–5791. [PubMed] [Google Scholar]
- 25.Schlereth B, Quadt C, Dreier T, Kufer P, Lorenczewski G, Prang N, Brandl C, Lippold S, Cobb K, Brasky K, Leo E, Bargou R, Murthy K, Baeuerle PA. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer Immunol Immunother. 2006;55:503–514. doi: 10.1007/s00262-005-0001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74:51–56. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Wolf IG, Lefterova P, Johnston V, Huhn D, Blume KG, Negrin RS. Propagation of large numbers of T cells with natural killer cell markers. Bri J Haematol. 1994;87:453–458. doi: 10.1111/j.1365-2141.1994.tb08297.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–1679. [PubMed] [Google Scholar]
- 29.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal DM, Weiner GJ, Weiner LM. Bispecific antibodies in cancer therapy. Curr Opin Immunol. 1999;11:558–562. doi: 10.1016/S0952-7915(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 31.Strasser A. Apoptosis: death of a T cell. Nature. 1995;373:385–386. doi: 10.1038/373385a0. [DOI] [PubMed] [Google Scholar]
- 32.van Spriel AB, van Ojik HH, van De Winkel JG. Immunotherapeutic perspective for bispecific antibodies. Immunol Today. 2000;21:391–397. doi: 10.1016/S0167-5699(00)01659-5. [DOI] [PubMed] [Google Scholar]
- 33.Verneris MR, Ito M, Baker J, Arshi A, Negrin RS, Shizuru JA. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol Blood Marrow Transplant. 2001;7:532–542. doi: 10.1016/S1083-8791(01)70014-6. [DOI] [PubMed] [Google Scholar]
- 34.Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- 35.Verneris MR, Kornacker M, Mailander V, Negrin RS. Resistance of ex vivo expanded CD3+CD56+ T cells to Fas-mediated apoptosis. Cancer Immunol Immunother. 2000;49:335–345. doi: 10.1007/s002620000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeidler R, Mysliwietz J, Csanady M, Walz A, Ziegler I, Schmitt B, Wollenberg B, Lindhofer H. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261–266. doi: 10.1054/bjoc.2000.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]