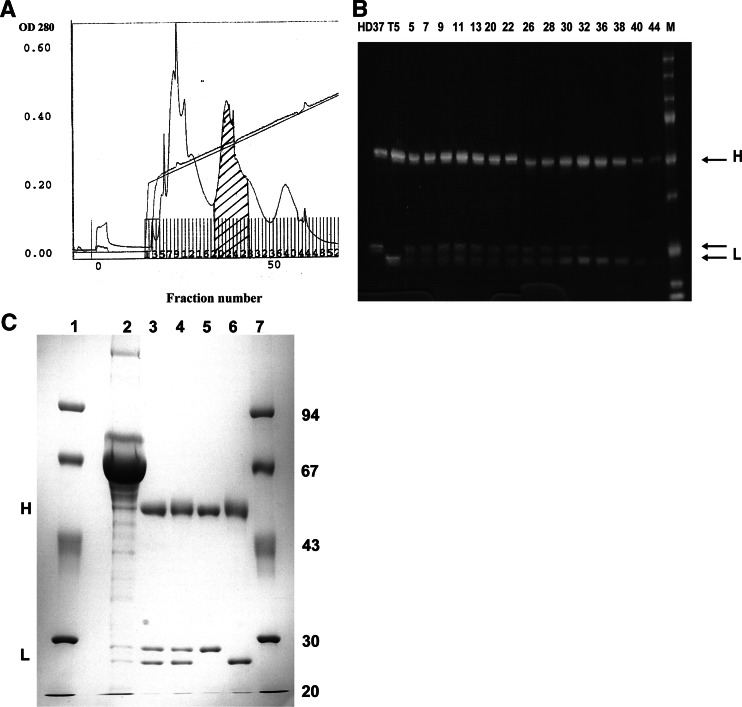
Fig. 1.
a Purification of bsAb HD37xT5.16 by FPLC separation on a Mono Q column. Elution was performed using a sodium chloride gradient in Tris–HCl (pH 8.0). The hatched peak contained the bispecific antibody. b SDS-PAGE analysis under reducing conditions of HD37xT5 16 preparations in comparison with parental monoclonal antibody. Lanes HD37 and T5, parental mAbs; lane M, molecular weight marker; lanes 5–44, FPLC-eluted fractions. Fractions 20 to 27 contained the bsAb and were pooled. c SDS-PAGE analysis under reducing conditions of HD37xT5.16 bsAb before and after purification. Lanes 1 and 7, molecular weight marker; lane 2, quadroma supernatant; lane 3, protein A-Sepharose CL-4B purified material; lane 4, FPLC-purified bsAb pool; lane 5, parental mAb HD37; lane 6, parental mAb T5.16. The positions of immunoglobulin heavy (H) and light (L) chains are indicated