Abstract
Background
Superficial bladder cancers are usually managed with transurethral resection followed by the intravesical administration of Bacillus Calmette-Guerin which requires major histocompatibility complex (MHC) class I expression on cancer cells. Since cancer cells often loose MHC expression, a novel immunotherapy such as MHC-unrestricted γδ T cell therapy is desired.
Objective
To clarify the relationship between the expression of MHC class I and clinicopathological features in bladder cancer patients, and investigate the effects of the administration of intravesical γδ T cells on bladder cancer.
Methods
Samples from 123 patients who had undergone either transurethral resection or radical cystectomies were examined for MHC expression and the relationship between this and the clinicopathological features was analyzed statistically. The in vitro and in vivo effects of γδ T cells expanded by zoledronic acid (ZOL) against several types of cancer cell line and an orthotopic bladder cancer murine model which was pretreated with ZOL were investigated.
Results
MHC-diminished superficial bladder cancer was significantly more progressive than MHC-conservative bladder cancer (P = 0.047). In addition, there was a significant association between diminished MHC expression and poor disease free survival (P = 0.041) and overall survival (P = 0.018) after radical cystectomy. In vitro, all of the cell lines pretreated with 5-μM ZOL showed a marked increase in sensitivity to lysis by γδ T cells. Moreover, intravesical administration of γδ T cells with 5-μM ZOL significantly demonstrated antitumor activity against bladder cancer cells in the orthotopic murine model (P < 0.001), resulting in prolonged survival.
Conclusion
The present murine model provides a potentially interesting option to develop immunotherapy using γδ T cells for bladder cancer in human.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-008-0571-9) contains supplementary material, which is available to authorized users.
Keywords: Bladder cancer, Intravesical administration, Major histocompatibility complex, γδ T, Zoledronic acid
Introduction
Superficial bladder cancers, which comprise approximately 70% of bladder cancers at initial diagnosis are usually managed with transurethral resection (TUR), followed by the intravesical administration of agents such as mitomycin C, adriamycin, and Bacillus Calmette-Guerin (BCG) [1, 2]. Among these intravesical agents, BCG is considered to be the most effective for the eradication and prophylaxis of recurrent superficial bladder cancer, including carcinoma in situ (CIS) and residual cancers [1–3]. BCG is believed to initially attach to bladder surfaces that are coated with extracellular matrix protein and subsequently cause a local inflammatory reaction in the bladder mucosa, characterized by large numbers of T cells including CD4 positive helper T-lymphocytes, CD8 positive cytotoxic T-lymphocytes (CTL), and macrophages [4]. The CTL-mediated antitumor effect is one of the major contributors of BCG therapy; therefore, preservation of major histocompatibility complex (MHC) class I should be necessary for its efficacy. Indeed, Kitamura et al. [5] recently reported that expression of MHC class I molecules on tumor cells contributes significantly to the therapeutic effect of BCG immunotherapy on bladder cancer. Although information on MHC class I expression patterns in bladder cancer is limited, down-regulation of MHC class I molecules in tumor cells is thought to be an important mechanism of tumor escape from immune surveillance [5, 6].
One of the T-lymphocyte subsets, γδ T cells, displays MHC-unrestricted cytotoxicity that is reminiscent of natural killer (NK) activity [7, 8]. Currently, γδ T cells are considered to represent a promising new concept in immunotherapy. Recently, we reported that zoledronic acid (ZOL), the most potent bisphosphonate, induced a significant dose-dependent expansion of γδ T cells both in vitro and in vivo, mainly to the Vγ 9Vδ 2 subset [9]. These observations have recently facilitated the development of novel auto-immunotherapeutic approaches using in vitro expanded γδ T cells from patients and have already yielded encouraging preliminary results [10].
In the present study, we attempted to evaluate the use of MHC class I expression as a prognostic factor for bladder cancer and also investigated the use of intravesical γδ T cells as a possible MHC-unrestricted therapeutic tool against bladder cancer. γδ T cells, which express many of the NK receptors including inhibitory types that recognize HLA class I such as NK inhibitory receptors (KIR) [11]. This may be advantageous because, the disrupting interactions of KIR with their ligands on tumor cells may enhance antitumor responses mediated by γδ T cells [12]. In addition, local administration may be more effective in treating cancer compared to systemic administration, owing to a favorable effecter/target cell (E/T) ratio. Indeed, some researchers have succeeded in performing local preclinical immunotherapy using γδ T cells in subcutaneous and intraperitoneal tumor models [13, 14]. Here, we performed intravesical immunotherapy using a bladder orthotopic model, which is close to the observations in a clinical setting.
Materials and methods
Patients and human samples
All 123 patients underwent either TUR or radical cystectomy and simultaneous bilateral pelvic lymph node dissection at the Department of Urology, Akita University School of Medicine between 1995 and 2003. Samples were fixed in formalin, embedded in paraffin, and sectioned for use in microscopic analysis. Informed consent was provided according to the Declaration of Helsinki. Clinical and pathological data were obtained by retrospective chart review, as previously described [15]. The 1997 TNM classifications were used for tissue staging. Ta is a noninvasive papillary carcinoma and T1 means that the tumor invades sub epithelial connective tissue.
Animals, cell lines, and reagents
Approval for these studies was obtained from the institutional review board at Kyoto University Hospital. Specific pathogen-free 6–8-week-old male Balb/c severe combined immunodeficiency (SCID) mice were used (SLC, Kyoto, Japan). The human bladder cancer cell line UM-UC-3 and KU-7, the small cell lung cancer cell line SBC-5, the non-small lung cancer cell line A549, the fibrosarcoma cell line HT1080, and the mesothelioma cell line 211H were obtained from the American Tissue Type Culture collection (Rockville, MD). ZOL and interleukin-2 (IL-2) were obtained from Novartis Pharma AG (Basel, Switzerland) and Shionogi (Osaka, Japan), respectively. UM-UC-3 cells were stably transfected with the pGL3-control vector (Promega, Madison, WI) and pSV2Neo (the American Type Culture Collection) and denoted as UM-UC-3Luc. These cells were maintained as described previously, to use for the in vivo imaging system (IVIS; Xenogen, Alameda, CA), which detects luciferase (Luc) expression [16]. We confirmed that there was no difference in in vitro proliferation between parental UM-UC-3 and UM-UC-3Luc.
Immunohistochemical staining
Immunohistochemical staining was performed by the conventional avidin–biotin–peroxidase complex method (ABC-Elite, Vector Labs), as described previously [16]. Anti-human MHC-1 monoclonal antibody EMR8-5 (Hokudo, Sapporo, Japan) was used at 1:100 dilution for the evaluation of patients samples. Sections were counterstained with hematoxylin and mounted. Normal mouse IgG was used as a negative control.
Immunohistochemical evaluation
All of the specimens were reviewed independently using light microscopy by investigators who were blind to the clinicopathologic data (TY and NT). Staining results were assessed in a semi-quantitative fashion by two independent investigators, as described previously [5]. Briefly, immunoreactivity for MHC class I was categorized on a scale of 0–3 as follows: 0, undetectable staining; 1, incomplete membrane staining in more than 20% of the tumor cells; 2, moderate to complete staining in the cytoplasm of the tumor cells; 3, complete membrane staining in more than 80% of the tumor cells [5]. MHC class I expression was then classified as negative (scores 0, 1, 2) or positive (score 3) [5].
Western blot analysis
Western blotting analysis was performed as described previously [17]. Equal amounts of protein extracts (50 μg) from peripheral blood mononuclear cells (PBMCs), UM-UC-3 or KU-7 were subjected to 12.5% polyacrylamide gel under denaturing conditions (SDS-PAGE) and then electroblotted onto a PVDF membrane (Millipore, Tokyo, Japan), as described previously [17]. Anti-human MHC-1 monoclonal antibody EMR8-5 with a 1:1,000 dilution was used as the primary antibody. Anti-human vinculin (Abcam, Tokyo, Japan) was used as a loading control.
Human γδ T cell preparations and culture
Informed consent was obtained for the collection of peripheral blood from healthy volunteers. Human γδ T cells were prepared as described previously [9]. Briefly, PBMCs were separated individually from blood samples donated from five healthy volunteers using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). PBMCs (1 × 105 cells) were stimulated with 5 μM of ZOL and cultured in 24-well round-bottom microtiter wells (Nunc, Wiesbaden, Germany) for 14 days at 37°C. On days 2, 6 and 10, 50 units/mL IL-2 were added to the culture. These ex vivo expanded γδ T cells were enriched for the cytotoxicity assay both in vitro and in vivo using a magnetic activated cell sorting system (Miltenyi Biotech, Bergisch Gladbach, Germany) to exclude αβ T cells, as described previously [9]. After the enrichment, the percentage of γδ T cells was always more than 99.8% (data not shown).
In vitro cytotoxicity assay
The in vitro cytotoxicity of γδ T cells from three healthy volunteers against SBC-5, HT-1080, UM-UC-3, 211H, and A549 was examined quantitatively using a standard 51Chromium (51Cr) releasing assay [9]. Briefly, 100-μl aliquots of each cell line that had been pretreated with 5-μM ZOL for 12 h were added to 51Cr for the final 2 h and then washed 3 times. The cells were then incubated for 4 h with ex vivo expanded γδ T cells at an E/T ratio 10:1, the supernatants were collected, and the radioactivity of 51Cr released from target cells was measured in a gamma counter (Wallac, Gaithersburg, MD). The maximum 51Cr release was determined in target cells treated with Triton X-100 at final concentration of 0.5%. The cytotoxicity was defined as the cell lysis percent according to the formula: cell lysis % = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
In vivo effects of γδ T cells in the orthotopic bladder cancer murine model
In order to establish the orthotopic bladder cancer models, Luc-labeled bladder cancer cells (2 × 106) were implanted into the murine bladder cavity via 24-gauge angiocatheters (Termo, Tokyo, Japan), as described previously [16]. Male BALB/c SCID mice were intravesically administered 1 × 107 UM-UC-3Luc cells. Mice were randomized into four groups: (i) untreated mice, (ii) mice treated with 5-μM ZOL (200 μl), (iii) mice treated with 1 × 107 purified γδ T cells and (iv) mice treated with 5-μM ZOL (200 μl) and 1 × 107 purified γδ T cells. Randomization was performed 7 (first experiment) and 3 days (second experiment) after the cancer cell transplantation and each group contained 8 mice. 100 μL of 5-μM ZOL for 3 h and/or 1 × 107 γδ T cells were administered for 5 sequential days from day 8 to day 12 for the first experiment and from day 4 to day 8 for the second experiment by the transurethral and intravesical method. The effect of γδ T cells on UM-UC-3Luc was monitored by IVIS (total number of photons), as described previously [18]. To examine how many of the γδ T cells survive after 3-h incubation in the bladder, 1 × 107 γδ T cells were administrated by the transurethral and intravesical method to intact bladders. γδ T cell variability was examined after 3-h incubation in the bladders by the trypan blue dye exclusion method. In addition, to examine how γδ T cells infiltrate healthy bladder epithelium and/or cancer lesions, 1 × 107 γδ T cells were also administrated by the transurethral and intravesical method to orthotopic bladder cancer mice models that had been 21 days earlier inoculated with 1 × 107 UM-UC-3Luc cells. After treatment with ZOL and γδ T cells, the bladders were dissected (each group, n = 3) and examined histologically after hematoxylin–eosin staining and immunohistochemically with anti-human CD3 (Novocastra Laboratories Ltd, Newcastle, UK) by light microscopy.
Statistical analysis
All data were entered into an access database and analyzed using Excel 2000 (Microsoft Co., Tokyo, Japan) and SPSS (version 10.0, SPSS, Inc., Tokyo, Japan) software; a probability (P) of <0.05 was required for statistical significance. Chi-square analysis was used for the variables of sex, age, configuration, grade, stage, and lymphatic and vascular involvement. Recurrence of the superficial cancers was defined as positive cytology or a pathologically proven tumor. Recurrence of invasive cancers was defined as clinically detected tumors, chiefly by imaging. Kaplan–Meier analysis was used to estimate the cumulative recurrence free survival, cause-specific survival, and overall survival rates, and the log-rank test was employed to correlate differences in patient survival with the staining intensity of MHC class I. Hazard ratios (HRs) and 95% confidence intervals (CIs) for disease free survival were assessed by the Cox proportional hazard regression model. The influence of the treatment on the growth of bladder cancers was analyzed by the Student’s t test.
Results
Relationship between the expression of MHC class I and clinicopathological features in patients with superficial bladder cancer
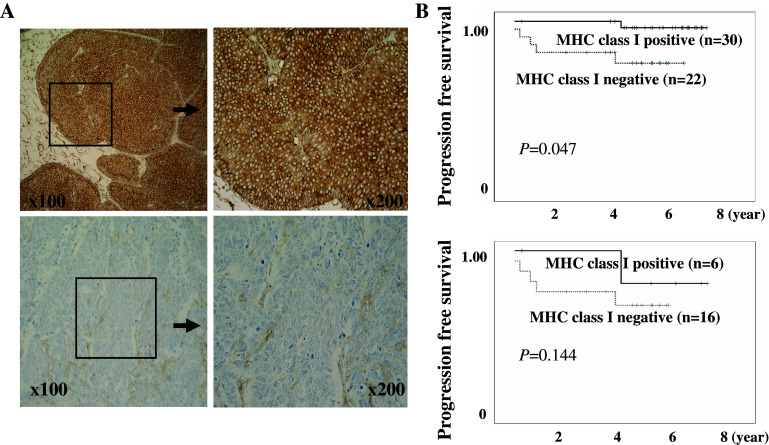
We initially tested whether the immunohistochemical levels of MHC class I expression correlated with the clinicopathological features, recurrence and progression free survival of patients with superficial bladder cancer. The histologically high grade, T1, and CIS co-existence bladder cancers demonstrated diminished MHC class I expression which was significantly lower than that in the low grade, Ta, and free of CIS cancer patients (Fig. 1a; Table 1). Moreover, although the difference in recurrence free survival was not significant, patients with low MHC class I expression exhibited a significantly shorter progression free survival than patients with high MHC class I expression (Fig. 1b upper row; P = 0.047). In addition, of these patients, 22 underwent BCG instillation therapy. In general, patients with low MHC class I expression had a tendency to exhibit shorter progression free survival than the patients with high MHC class I expression, although this difference was not statistically significant (Fig. 1b lower row, P = 0.144).
Fig. 1.
MHC class I expression correlates with the progression of superficial bladder cancer. a MHC class I expression in the tissues of superficial bladder cancer was immunohistochemically analyzed by the conventional avidin–biotin–peroxidase complex method using the anti-human MHC class I monoclonal antibody EMR8-5 at a 1:100 dilution. A strong immune-reaction in low grade bladder cancers (upper row) and a weak and diminished immune-reaction in high grade bladder cancers (lower row) were typically observed. Nuclear staining was performed with Mayer’s hematoxylin. b Comparison of the survival curves between patients with bladder cancers expressing high and low levels of MHC class I. Progression free survival curves for patients with superficial bladder cancer (upper row) and for those treated with intravesical BCG (lower row)
Table 1.
Relationship between the level of MHC class I expression and clinicopathological features of the patients with superficial bladder cancer
| Total (n = 52) | High (n = 30) | Low (n = 22) | P value | ||
|---|---|---|---|---|---|
| Age (mean SD) | 68.1 (15.4) | 67.1 (16.9) | 69.4 (13.4) | 0.98 | |
| Sex | |||||
| Male | 41 | 23 | 18 | 0.65 | |
| Female | 11 | 7 | 4 | ||
| Number of tumors | |||||
| CIS | 11 | 3 | 8 | 0.50 | |
| Solitary | 12 | 7 | 5 | ||
| Multiple | 27 | 20 | 7 | ||
| Grade | |||||
| Well/mod | 31 | 23 | 8 | 0.0034 | |
| Poor | 21 | 7 | 14 | ||
| Depth of invasion | |||||
| CIS | 11 | 3 | 8 | 0.0013 | |
| Ta | 27 | 24 | 3 | ||
| T1 | 14 | 3 | 11 | ||
| Recurrence | |||||
| Negative | 31 | 18 | 13 | 0.95 | |
| Positive | 21 | 12 | 9 | ||
| Progression | |||||
| Negative | 47 | 29 | 18 | 0.072 | |
| Positive | 5 | 1 | 4 | ||
|
Follow-up in month (mean SD) |
43.0 (28.3) | 44.9 (31.5) | 40.3 (24.5) | 0.56 | |
Relationship between the expression of MHC class I and clinicopathological features in patients with invasive bladder cancer
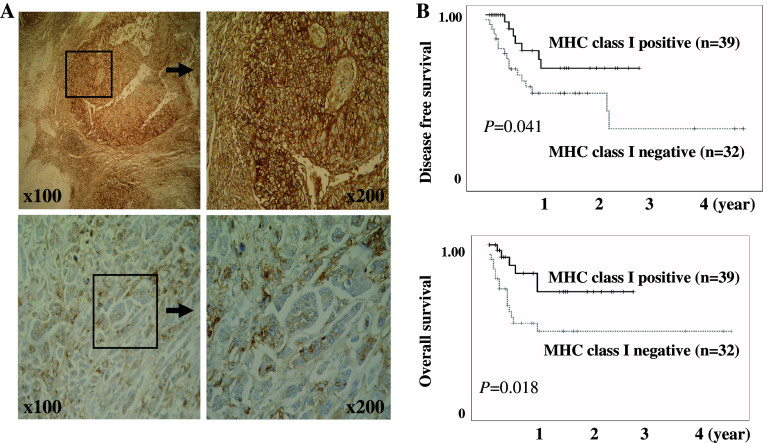
Next, we tested whether immunohistochemical levels of MHC class I expression were related to various clinicopathological features and survival rates in patients with invasive bladder cancer. The lymph node involvement and lymphatic invasive bladder cancers demonstrated diminished MHC class I expression by immunohistochemistry, compared to the non-lymph node involvement and non-lymphatic invasive cancers (Fig. 2a; Table 2). Moreover, the patients with low MHC class I expression experienced a significantly shorter disease free survival time and overall survival than patients with high MHC class I expression (Fig. 2b upper and lower row, P = 0.041, 0.018, respectively). A Cox proportional hazard regression analysis indicated that perivesical invasion (≥pT3a), high grade, lymph node involvement (pN+), and MHC diminishing were associated with poor disease free survival. However, a multivariate analysis using all of these clinicopathological and molecular factors indicated that only lymph node involvement was a significantly unfavorable prognostic factor independent of other factors (P = 0.021). These results suggest that bladder cancers with diminished MHC class I expression are biologically aggressive and treatment-resistant tumors.
Fig. 2.
MHC class I expression correlates with the progression of invasive bladder cancer. a MHC class I expression in the tissues of invasive bladder cancer was immunohistochemically analyzed. A strong immune-reaction in moderate grade bladder cancers (upper row) and a weak and diminished immune-reaction in high grade bladder cancers (lower row) were typically observed. Micropapillary growth pattern of the high grade bladder cancers also demonstrated a diminished immune-reaction (lower row). Numbers indicate original magnifications. b Comparison of the survival curves between patients with bladder cancers expressing high and low levels of MHC class I. Disease free survival curve (upper row) and overall survival curve (lower row) for invasive bladder cancer after radical cystectomy
Table 2.
Relationship between the level of MHC class I expression and clinicopathological features of the patients with invasive bladder cancer
| Total (n = 71) | High (n = 39) | Low (n = 32) | P value | ||
|---|---|---|---|---|---|
| Age (mean SD) | 67.0 (12.6) | 67.4 (14.2) | 66.4 (11.0) | 0.96 | |
| Sex | |||||
| Male | 55 | 33 | 22 | 0.11 | |
| Female | 16 | 6 | 10 | ||
| Configuration | |||||
| Papillary | 14 | 9 | 5 | 0.52 | |
| Non-papillary | 53 | 29 | 24 | ||
| Flat | 4 | 1 | 3 | ||
| Number of tumors | |||||
| Solitary | 24 | 12 | 12 | 0.41 | |
| Multiple | 43 | 26 | 17 | ||
| Flat | 4 | 1 | 3 | ||
| Grade | |||||
| Well/mod | 10 | 8 | 2 | 0.085 | |
| Poor | 61 | 31 | 30 | ||
| Depth of invasion | |||||
| <T2 | 26 | 15 | 11 | 0.72 | |
| >T3 | 45 | 24 | 21 | ||
| Lymph node involvement | |||||
| Negative | 18 | 6 | 12 | 0.027 | |
| Positive | 47 | 30 | 17 | ||
| Unknown | 6 | 3 | 3 | ||
| Lymphatic invasion | |||||
| Negative | 31 | 22 | 9 | 0.017 | |
| Positive | 40 | 17 | 23 | ||
| Venous invasion | |||||
| Negative | 41 | 26 | 15 | 0.093 | |
| Positive | 30 | 13 | 17 | ||
| Follow-up in month (mean) | 28.7 (25.8) | 27.3 (20.0) | 30.2 (30.2) | 0.67 | |
Ex vivo-expanded γδ T cells show cell dose-dependent cytotoxic activity against various cancer cell lines
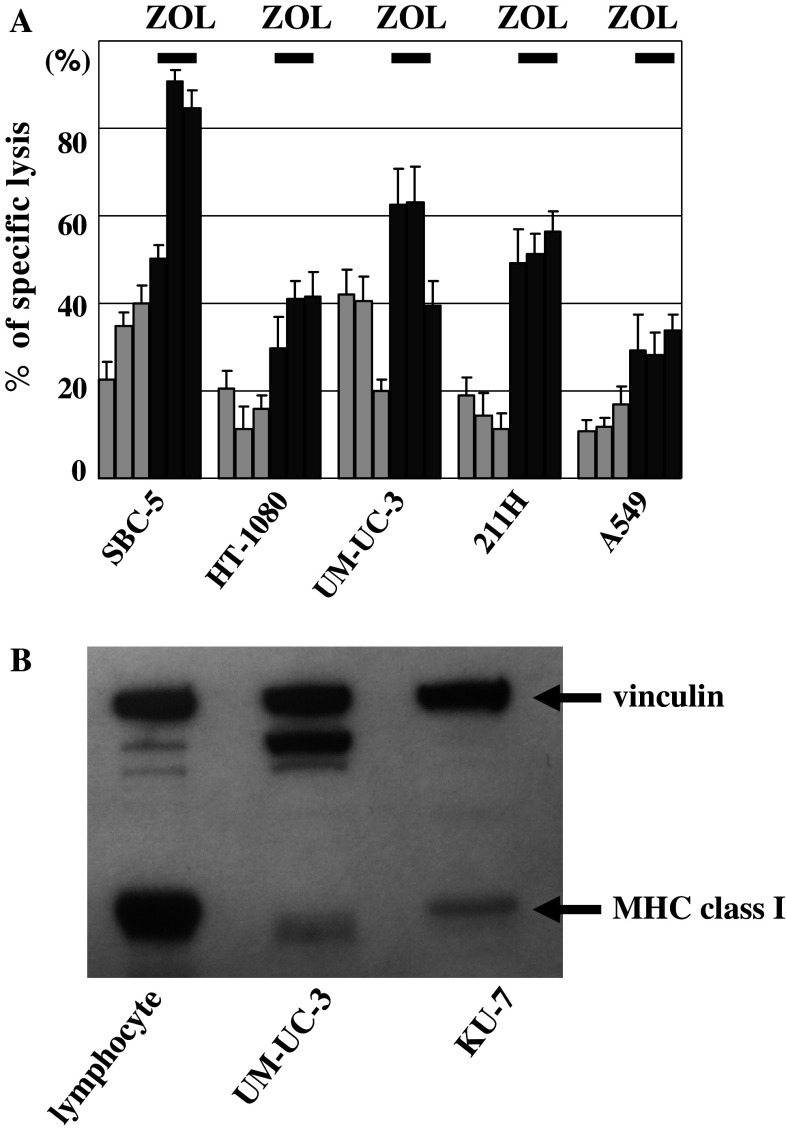
In order to overcome MHC class I deletion, we investigated the use of γδ T cells as a potential therapeutic tool. We investigated the cytotoxic activity of ex vivo-expanded γδ T cells at an E/T ratio of 10:1 against various cancer cell lines. The average cytotoxicity of the γδ T cells from three healthy volunteers with and without pre-treatment of 5 μM of ZOL was 75.2 and 32.4% for SBC-5 small cell lung cancer cells, respectively, 37.3 and 15.9% for HT-1080 fibrosarcoma cells, respectively, 52.2 and 14.8% for UM-UC-3 bladder cancer cells, respectively, 55.0 and 34.2% for 211H mesothelioma cells, respectively, and 30.4 and 13.2% for A549 non-small cell lung cancer cells, respectively (Fig. 3a). The differences in cytotoxicity between cells with no pretreatment and those pretreated with a low dose ZOL were significant for all of the cells examined (P < 0.001 for all cells). Thus, γδ T cells kill various cancer cell lines and a low dose of ZOL can augment their cytotoxic effect.
Fig. 3.
In vitro combined effects of γδ T cells with ZOL on various cancer cells and their MHC class I expression. a Augmentation of the cytotoxic activity of the ex vivo-expanded γδ T cells by a low dose of ZOL (5 μM) in various cancer cell lines. In vitro cytotoxicity of γδ T cells from 3 healthy volunteers against SBC-5, HT-1080 UM-UC-3, 211H, and A549 was examined quantitatively by a standard 51Chromium (51Cr) releasing assay. Each value indicates the mean ± SEM (n = 6). b MHC class I expressions of human normal PBMNCs, UM-UC-3 and KU-7. The data shown are representative of 3 independent experiments
γδ T cells in the bladder
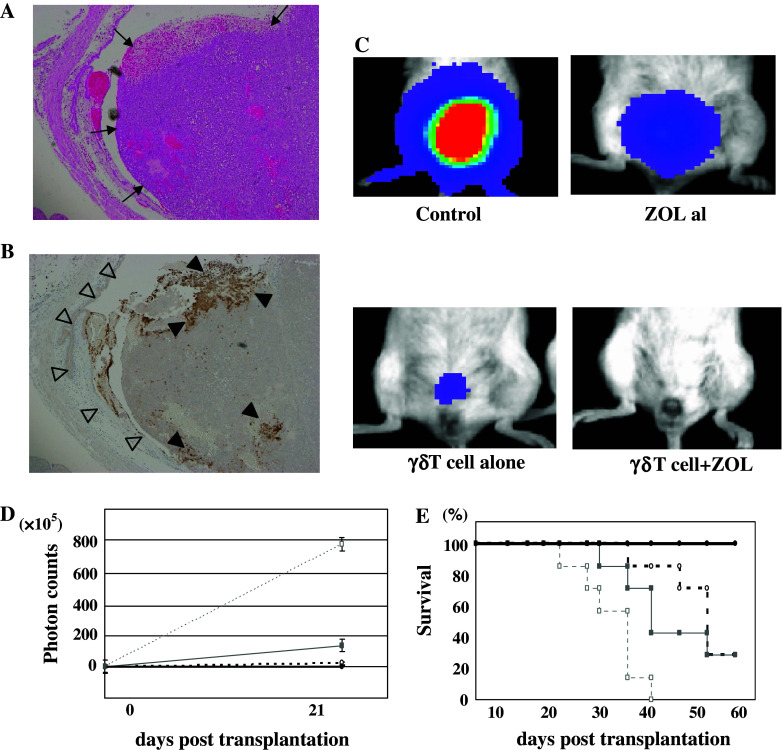
A total of 86.5 ± 6.3% of γδ T cells survived after 3-h incubation in the bladder (n = 5). UM-UC-3 which was used for in vivo experiments revealed less MHC class I expression than normal human PBMCs and KU-7 cells (Fig. 3b). γδ T cells massively infiltrated tumor in the bladder, while few γδ T cells infiltrated the healthy bladder epithelium (Fig. 4a, b). These findings suggested that the treatment of 100 μL of 5-μM ZOL for 3 h and/or 1 × 107 γδ T cells might show in vivo growth inhibitory effects in the orthotopic murine bladder cancer models.
Fig. 4.
In vivo effects of intravesical administrated γδ T cells. After 3-h incubation with ZOL and γδ T cells, the bladders was occupied by a large tumor (↑) were dissected and examined histologically with hematoxylin-eosine staining (a) and immunohistochemically with anti-human CD3 antibody (b). Although the region of healthy bladder epithelium (triangle) was not infiltrated by γδ T cells, the tumor was massively infiltrated by γδ T cells indicated by human CD3 positivity (filled triangle). Original magnification ×16. The data shown are representative of 3 independent experiments. c In vivo effects of intravesical γδ T cells in the orthotopic bladder cancer murine model. Typical images of the respective mice not treated or treated with a low dose of ZOL alone, γδ T cells alone, or γδ T cells and a low dose of ZOL, were observed by IVIS. d The growth curves of orthotopically transplanted UM-UC-3LUC were measured by IVIS. The anti-cancerous effect of intravesical ex vivo-expanded γδ T cells was demonstrated in vivo: no treatment, open square; treatment with a low dose of ZOL alone, closed square; treatment with γδ T cells alone, open circle; treatment with γδ T cells and a low dose of ZOL, closed circle. e The survival curves of mice not treated or treated with γδ T cells alone, a low dose of ZOL alone, or γδ T cells and a low dose of ZOL. Survival of the orthotopic mice was improved by the intravesical administration of ex vivo-expanded γδ T cells: no treatment, open square; treatment with a low dose of ZOL alone, closed square; treatment with γδ T cells alone, open circle; treatment with γδ T cells and a low dose of ZOL, closed circle
In vivo growth inhibition of human bladder cancer in orthotopic murine models by transurethral administration of ex vivo-expanded human γδ T cells
We introduced UM-UC-3LUC cells into the bladder, and observed their bioluminescence by IVIS. Bioluminescence was not detectable the following day, but was faintly detected 3 days later. In the initial experiment (the first experiment), we randomized these mice on day 8 after transplantation and administered the respective intravesical agent for 5 sequential days starting on day 9. There was no difference between these groups, possibly due to a low E/T ratio. In the second experiment, we divided the mice into 4 groups at day 3 after transplantation. We investigated the growth inhibitory effect of the human γδ T cells with or without a low dose of ZOL (5 μM) in vivo. Figure 4c shows typical images at day 21 taken by IVIS and the growth curves of the respective transplanted cancers in murine bladder (Fig. 4d). Although equivalent numbers of cancer cells were injected, cancer growth rates differed among the treatment groups. Photon emissions from mice treated with both human γδ T cells and a low dose of ZOL were significantly lower than those from mice in the non-treatment groups (P < 0.001). Moreover, the photon emissions from mice treated with human γδ T cell and a low dose of ZOL were significantly lower than those of the mice from either the γδ T cells alone or a low dose of ZOL alone (P = 0.048, P < 0.001, respectively).
The mucosal surfaces of the γδ T cell treated murine bladders did not show apparent injury and there were no microscopic differences in the non-cancerous bladder mucosa among the treated and non-treatment mice (Fig. 4a). We found no differences in the body weight among the groups of mice. Furthermore, the mice treated with the combination of γδ T cells and a low dose of ZOL showed apparently better survival compared with the non-treated, γδ T cell alone or a low dose of ZOL alone treatment groups (Fig. 4e). The median survival time was 31, 58, and 44 days for mice not treated, treated with γδ T cell alone, and treated with a low dose of ZOL alone, respectively. The median survival time was not reached by mice treated with the combination of γδ T cell and a low dose of ZOL until the end of the experiment.
A combination of γδ T cells and a low dose of ZOL inhibited the growth of bladder cancers not only in vitro, but also in vivo, and the duration of survival was also significantly prolonged by these treatments without any severe adverse effects in the murine model.
Discussion
In this study, we demonstrated that a loss of MHC class I expression in bladder cancer is a poor prognostic factor both for the progression of superficial cancers and the survival of invasive bladder cancers. Approximately 40–90% of human cancers derived from various MHC class I positive tissues have been reported to be MHC class I deficient [6]. Bladder cancers exhibiting a down-regulation of MHC class I expression acquire the ability to escape from T cell-mediated immune responses, consequently resulting in tumor development, progression, and a poor outcome; these observations are in line with other recent reports [5, 6, 19]. Kitamura et al. [5] also clearly show that the expression of MHC class I molecules on bladder cancer cells contributes significantly to the therapeutic effect of BCG immunotherapy and to recurrence free survival of superficial bladder cancer. In contrast, Sharma et al. [20] reported that the presence of intratumoral CD8-positive tumor-infiltrating cytotoxic cells, not MHC class I expression, was significantly associated with clinical outcome among patients with invasive bladder cancer. These controversies might come from the sensitivity of the antibody against human MHC class I. However, their study is also in line with our observation that the CTL-mediated tumor immune microenvironment plays an important role in the tolerance of BCG therapy.
More than 50% of bladder cancers will recur intravesically, and 20–30% of the these cancers will develop to a higher grade and/or stage within the first 5 years of treatment, and progress to local invasive cancers [20]. In order to avoid progression to an invasive cancer, we have investigated the use of small interfering RNA (siRNA) targeting the polo like kinase-1 (PLK-1) gene as a novel therapeutic approach against bladder cancer [16]. We have demonstrated that intravesical administration of PLK-1 targeted siRNA/cationic liposomes inhibited cancer growth in murine orthotopic bladder cancer models and that the transurethral siRNA therapy could overcome the drug delivery system problem of siRNA delivery.
Molecular targeted therapies are very promising especially for the hematological disorders such as Abl tyrosine kinase inhibitors for chronic myeloid leukemia [21]. However, the efficacy of molecular targeted therapy for solid malignancy remains to be unsolved. A recent topic for urological oncology is the success of sunitinib, which is a novel specific inhibitor of the receptor tyrosine kinases, and anti-angiogenic agents for metastatic renal cell cancer. Although progression free survival was longer and response rates were higher in patients with metastatic renal cell cancer who received sunitinib than in those receiving interferon-α, complete remission was seldom seen even in patients who received sunitinib [22]. The pathogenesis of solid malignancies such as bladder cancer is complicated; therefore, the efficacy of simple gene target therapy is doubtful. We attempted to perform immunotherapy in combination with molecular targeted therapy. We investigated the use of the anti-cancer action of γδ T cells, which represent a minor subset of human peripheral T cells (1–10%) and contribute to the host immune defense in a different way to CTLs [8].
In this study, we initially demonstrated that γδ T cells, proliferated by bisphosphonates, demonstrated active anti-cancerous effects against various cancer cells in vitro; this is consistent with previous reports [23]. Kato et al. [23] clearly demonstrated that bladder cancer cells can efficiently present bisphosphonate and pyrophosphomonoester compounds to γδ T cells, inducing specific proliferation and interferon-γ production. Internalization of bisphosphonates by cancer cells led to rapid inhibition of farnesyl pyrophosphate (FPP) synthase, resulting in intracellular accumulation of isopentenyl pyrophosphate (IPP) upstream of FPP synthase in the mevalonate pathway (Supplemental Fig. 1) [7, 24]. Accumulated IPP acts as a powerful danger signal that activates the immune response and as such might represent a novel target for tumor immunotherapy [7, 9]. We have reported that the tumor killing mechanism of γδ T cells depends mainly on direct contact involving a perforin-dependent cytolytic pathway [9, 25]. Consequently, we advanced to in vivo experiments.
Here, we have demonstrated that intravesical administration of γδ T cells with a low dose of ZOL inhibited cancer growth in a murine orthotopic bladder cancer model. Previously, we have confirmed that a high dose of intravesical ZOL resulted in growth inhibition of bladder cancer without any severe side effects [26]. Most current immunotherapeutic approaches such as BCG therapy, which is currently the most effective agent for bladder cancer treatment aim to induce an antitumor response via stimulation of the adaptive immune system, which is dependent on MHC-restricted CTLs. In contrast, γδ T cells from healthy volunteers, which were proliferated ex vivo, were able to function in an MHC-unrestricted manner and thus, MHC-unrestricted γδ T cells represent a promising new concept in immunotherapy. The initial failure of the first experiment was probably due to an insufficient E/T ratio; therefore, in the second experiment we administrated γδ T cells earlier. Improvement of the E/T ratio gave us satisfactory results. Although we have not yet investigated whether patient’s γδ T cells behave similarly as those from healthy volunteers, patient’s γδ T cells might have antitumor effects. Kobayashi et al. [27] reported that adoptive immunotherapy using in vitro-activated autologous γδ T cells induced antitumor effects in patients with advanced renal cell carcinoma after radical nephrectomy. Taken together, we hypothesize that a combination of molecular targeted therapy such as PLK-1 siRNA and this novel MHC class I-unrestricted immunotherapy may overcome the progression of bladder cancer.
In conclusion, we believe that the antitumor effect of intravesical autologous γδ T cells is an attractive tool for use as a novel immunotherapy and may cause a break-through in clinical applications of cell therapeutics. The efficacy and safety of intravesical γδ T cells should be verified by early phase clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1 Schematic diagram of the mevalonate pathway and site of action of ZOL (PDF 15 kb)
Acknowledgments
We thank Ms Yoko Nakagawa, Yoko Mitobe and Yukiko Sugiyama for their skillful technical assistance. This work was partly supported by the Shimadzu Science Foundation, the Sagawa Foundation for Promotion of Cancer Research, Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, and the COE program of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- BCG
Bacillus Calmette-Guerin
- CIs
Confidence intervals
- CIS
Carcinoma in situ
- 51Cr
51 Chromium
- CTL
Cytotoxic T-lymphocytes.
- E/T
Effecter/target cell
- IL-2
Interleukin-2
- IVIS
In vivo imaging system
- HRs
Hazard ratios
- Luc
Luciferase
- MHC
Major histocompatibility complex
- NK
Natural killer
- PBMCs
Peripheral blood mononuclear cells
- SCID
Severe combined immunodeficiency
- siRNA
Small interfering RNA
- TUR
Transurethral resection
- ZOL
Zoledronic acid
Footnotes
T. Yuasa and K. Sato contributed equally to the study.
References
- 1.Jones SJ, Campbell SC. Non-muscle invasive bladder cancer. In: Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ, editors. Campbell-Walsh Urology. 8. New York: Saunders; 2006. pp. 2447–2467. [Google Scholar]
- 2.Gee J, Sabichi AL, Grossman HB. Chemoprevention of superficial bladder cancer. Crit Rev Oncol Hematol. 2002;43:277–286. doi: 10.1016/S1040-8428(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Laudone VP, Badalament RA, Oettgen HF, Sogani PC, Freedman BD, Melamed MR, Whitmore WF., Jr Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J Clin Oncol. 1988;6:1450–1455. doi: 10.1200/JCO.1988.6.9.1450. [DOI] [PubMed] [Google Scholar]
- 4.Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–1023. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura H, Torigoe T, Honma I, Sato E, Asanuma H, Hirohashi Y, Sato N, Tsukamoto T. Effect of human leukocyte antigen class I expression of tumor cells on outcome of intravesical instillation of bacillus calmette-guerin immunotherapy for bladder cancer. Clin Cancer Res. 2006;12:4641–4644. doi: 10.1158/1078-0432.CCR-06-0595. [DOI] [PubMed] [Google Scholar]
- 6.Bubenik J. Tumour MHC class I downregulation and immunotherapy. Oncol Rep. 2003;10:2005–2008. [PubMed] [Google Scholar]
- 7.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of γδ T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 10.Viey E, Laplace C, Escudier B. Peripheral gammadelta T-lymphocytes as an innovative tool in immunotherapy for metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2005;5:973–986. doi: 10.1586/14737140.5.6.973. [DOI] [PubMed] [Google Scholar]
- 11.Halary F, Peyrat MA, Champagne E, Lopez-Botet M, Moretta A, Moretta L, Vié H, Fournié JJ, Bonneville M. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–2821. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 12.Bakker AB, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J Immunol. 1998;160:5239–5245. [PubMed] [Google Scholar]
- 13.Kabelitz D, Wesch D, Pitters E, Zöller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767–6776. doi: 10.4049/jimmunol.173.11.6767. [DOI] [PubMed] [Google Scholar]
- 14.Lozupone F, Pende D, Burgio VL, Castelli C, Spada M, Venditti M, Luciani F, Lugini L, Federici C, Ramoni C, Rivoltini L, Parmiani G, Belardelli F, Rivera P, Marcenaro S, Moretta L, Fais S. Effect of human natural killer and gammadelta T cells on the growth of human autologous melanoma xenografts in SCID mice. Cancer Res. 2004;64:378–385. doi: 10.1158/0008-5472.CAN-03-1501. [DOI] [PubMed] [Google Scholar]
- 15.Ichimura Y, Habuchi T, Tsuchiya N, Wang L, Oyama C, Sato K, Nishiyama H, Ogawa O, Kato T. Increased risk of bladder cancer associated with a glutathione peroxidase 1 codon 198 variant. J Urol. 2004;172:728–732. doi: 10.1097/01.ju.0000130942.40597.9d. [DOI] [PubMed] [Google Scholar]
- 16.Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, Yokota A, Segawa S, Toda Y, Kageyama S, Yoshiki T, Okada Y, Maekawa T. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura S, Ito C, Jyoko N, Segawa H, Kuroda J, Okada M, Adachi S, Nakahata T, Yuasa T, Filho VC, Furukawa H, Maekawa T. Inhibition of leukemic cell growth by a novel anti-cancer drug (GUT-70) from Calophyllim brasiliense that acts by induction of apoptosis. Int J Cancer. 2005;113:158–165. doi: 10.1002/ijc.20505. [DOI] [PubMed] [Google Scholar]
- 18.Nogawa M, Yuasa T, Kimura S, Kuroda J, Sato K, Segawa H, Yokota A, Maekawa T. Monitoring luciferase-labeled cancer cell growth and metastasis in in vivo models. Cancer Lett. 2005;217:243–253. doi: 10.1016/j.canlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura H, Torigoe T, Honma I, Asanuma H, Nakazawa E, Shimozawa K, Hirohashi Y, Sato E, Sato N, Tsukamoto T. Expression and antigenicity of surviving, an inhibitor of apoptosis family member, in bladder cancer: implications for specific immunotherapy. Urology. 2006;67:955–959. doi: 10.1016/j.urology.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura S, Ashihara E, Maekawa T. New tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Curr Pharm Biotechnol. 2006;7:371–379. doi: 10.2174/138920106778521532. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 23.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human gammadelta T cells by nonpeptide antigens. J Immunol. 2001;167:5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 24.van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 25.Uchida R, Ashihara E, Sato K, Kimura S, Kuroda J, Takeuchi M, Kawata E, Taniguchi K, Okamoto M, Shimura K, Kiyono Y, Shimazaki C, Taniwaki M, Maekawa T. Gamma delta T cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem Biophys Res Commun. 2007;354:613–618. doi: 10.1016/j.bbrc.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Yuasa T, Nogawa M, Kimura S, Segawa H, Yokota A, Maekawa T. A third generation bisphosphonate, minodronic acid (YM529), successfully prevented the growth of bladder cancer in vitro and in vivo. Br J Cancer. 2006;95:1354–1361. doi: 10.1038/sj.bjc.6603423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1 Schematic diagram of the mevalonate pathway and site of action of ZOL (PDF 15 kb)