Abstract
Redirecting T cell effector functions towards pre-defined target cells represents an attractive concept in the adoptive immunotherapy of malignant diseases. Our understanding of the mechanisms of T cell activation and costimulation as well as the design of recombinant T cell receptors have made major progress in the last years. Translating recent concepts of T cell stimulation into recombinant protein design provides the basis to engineer T cells with both pre-defined specificity and costimulatory capacity in order to enhance anti-tumor immunity and to break tolerance. Dual signaling immunoreceptors providing the CD3ζ signal simultaneously with an appropriate costimulatory signal moreover allows to modulate the quality of the anti-tumor T cell response in a predicted fashion.
Keywords: Adoptive immunotherapy, Immunoreceptor, Recombinant TCR, Costimulation, CD28, OX40, 4-1BB
Introduction
T cells with specificity for tumor associated (self-)antigen (TAA) are frequently present in significant numbers in the peripheral blood of tumor patients and clones of cytotoxic T lymphocytes (CTLs) with specificity for autologous tumor cells can be derived thereof [1, 2]. These observations have attracted interest in the elimination of tumor cells by adoptively transferred, tumor-specific T cells. Consequently, tumor infiltrating lymphocytes (TILs) isolated from biopsy specimens eliminate tumor cells upon adoptive transfer demonstrating their in vivo effectivity and the feasibility of the strategy. Isolation of TILs in sufficient numbers and ex vivo propagation without loss of anti-tumor reactivity, however, turned out to be technically difficult and labor-intensive.
In this situation, considerable attention has been drawn to engineer T cells with pre-defined specificity by recombinant DNA technology. One strategy makes use of recombinant T cell receptors (TCRs) with defined specificity which are expressed in addition to the endogenous TCR on the surface of CTLs [3, 4]. An alternative strategy grafts specificity onto the recombinant TCR by fusion with an antibody derived binding domain. This approach is based on the observation that the majority of TAAs are defined by monoclonal antibodies and that immunoglobulins and TCRs exhibit significant spatial and conformational similarities. Whereas in early models the variable regions of the TCR α- and β-chains are replaced by antibody derived VH and VL regions for antigen binding [5], the concept became more feasible when Zelig Eshhar and coworkers [6] pioneered the immunoreceptor (“T-body- strategy which combines the antigen binding domain of a “single chain fragment of variable region-(scFv) antibody directly with the TCR associated intracellular signaling machinery (for review [7, 8]).
The strategy in targeting T cells towards defined target cells by recombinant TCRs or immunoreceptors has several advantages over antibody-based therapies. In particular, the strategy makes use of the autologous cellular defense system represented by effector cells that actively migrate to the target cell and penetrate tissues. In contrast to antibodies, the engineered effector cells persist and circulate over long periods of time, i.e. until up to one year or even longer, amplify by proliferation upon antigen encounter and interact with numerous target cells executing their cytolytic attack. Noteworthy, the immune reaction is supposed to be self-limiting since engineered T cells that do not interact with their specific antigen are expected to enter apoptosis. One of the major risks, on the other hand, is represented by an autonomous amplification of engineered T cells and by an unwanted auto-immune reaction towards healthy tissues. Whereas these prepresent general advantages and risks of cell versus antibody based therapies, it is still an ongoing debate which format of a recombinant TCR is the most efficient in T cell activation and with the lowest risk of unwanted side effects, e.g., dual versus single chain TCR, MHC restricted versus unrestricted antibody based immunoreceptor. We here focus on antibody derived immunoreceptors with particular emphases on the role of costimulation for redirecting and modulating T cell activation.
The immunoreceptor strategy
The antibody derived recombinant T cell receptor (immunoreceptor) is designed as a one-polypeptide chain composed of an extracellular binding and an intracellular signaling domain (Fig. 1). This design has several advantages for use in adoptive immunotherapy. Since the antigen binding domain is derived from an antibody, recombinant immunoreceptors can be generated that bind antigen of any chemical composition or conformation as far as an antibody exists. Indeed, recombinant immunoreceptors have been designed that target T cells towards carbohydrate antigens [9, 10]. The signaling moiety is preferentially derived from the CD3ζ chain of the TCR/CD3 complex or, alternatively, from the γ chain of the high affinity IgE Fc receptor (FcεRI) or from the lck signaling domain. The immunoreceptor tyrosine activation motifs (ITAMs) of the signaling domains become phosphorylated upon receptor crosslinking, thereby serving as specific adaptors for downstream signaling proteins of the TCR complex.
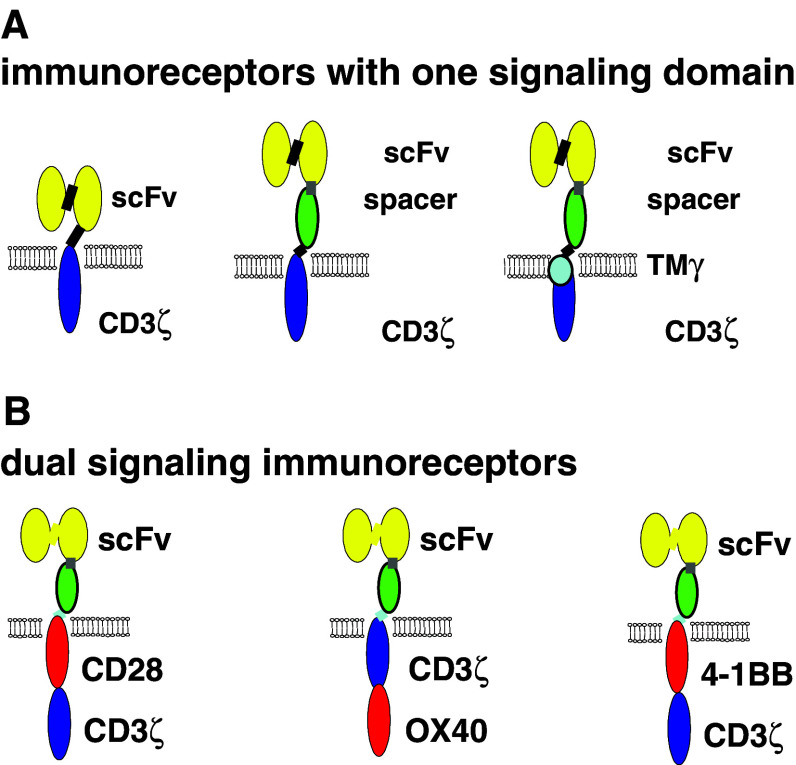
Fig. 1.
Schematic diagram depicting the modular composition of recombinant immunoreceptors. a The antigen binding domain (scFv) is linked either directly or via a spacer domain, preferentially the CH2CH3 domain of IgG1, to the transmembrane and intracellular signaling moiety; the latter is preferentially derived from the CD3ζ chain of the TCR/CD3 complex. b The primary signaling domain can be combined in series with a costimulatory domain within the same polypeptide chain giving rise to a combined, dual signaling immunoreceptor
When expressed in CTLs, antigen-mediated cross-linking of the immunoreceptor initiates T cell activation resulting in proliferation, cytokine secretion, and specific cytolysis of antigen-positive target cells. The efficiency of T cell activation depends on various parameters including the density of antigen expression, the targeted epitope and the position of the epitope within the antigen (unpublished results), as well as the density of immunoreceptors on the surface of the effector cells. The specificity of immunoreceptor mediated T cell activation is defined by the binding domain of the immunoreceptor because (1) T cells without immunoreceptor or equipped with an immunoreceptor of different specificity are not activated towards specific antigen-positive target cells, (2) antigen-negative cells do not drive receptor mediated T cell activation, and (3) triggered T cell activation can be blocked by an anti-idiotypic antibody directed towards the scFv domain of the immunoreceptor.
The feasibility of the strategy was repeatedly demonstrated in numerous in vitro mouse and human systems, in immunocompromised mice grafted with allogeneic tumors [9] and most recently in immunocompetent mice grafted with syngeneic tumors (A. Hombach, Chmielewski et al., unpublished results) [11, 12]. Noteworthy, T cells equipped with the appropriate immunoreceptor break tolerance towards autologous tumor cells in vitro, as repeatedly shown with patient’s T cells from the peripheral blood and primary human tumor cells isolated from a biopsy [13, 14].
The majority of immunoreceptors harbor antibody derived binding domains that recognize antigen independently of the MHC, thereby making immunoreceptor mediated T cell activation independent of processed and MHC presented antigens. However, there are also antibodies raised that recognize their antigen in the context of MHC. Immunoreceptors with such type of binding domain, thus, trigger T cell activation dependently on MHC presentation of antigen. It is so far not elucidated in detail whether immunoreceptors with a MHC dependent or an independent binding domain are superior in redirecting T cells in vivo. Some advantages of MHC independent antigen binding, however, are obvious, including targeting of unconventional T cell antigens like carbohydrates, targeting of cells even when the antigen is not processed or presented properly in the MHC, and recruiting of both CD8+ and CD4+ T cells by the same immunoreceptor [15]. The latter is of clinical relevance since both redirected CD4+ and CD8+ T cells execute granule-dependent cytolysis of target cells when appropriately stimulated via immunoreceptor [16].
CD28 costimulation modulates the immune response, but is not primarily required for the induction of cytolytic activities in pre-activated T cells
Optimal CD8+ T cell activation physiologically requires two distinct signals. The first is mediated by MHC class I-restricted, antigen-dependent TCR triggering and the second by antigen-independent costimulation. The latter is provided by a number of surface receptors, a prototype of which is CD28. CD28 signaling amplifies the primary T cell response, lowers the amount of antigen required to achieve full T cell activation and sustains prolonged polyclonal T cell expansion. The physiological situation is reflected by the requirement of costimulation to induce optimal activation of T cells upon immunoreceptor triggering. Naive T cells stimulated via CD3ζ signaling immunoreceptor, in contrast to pre-activated T cells, are not fully activated and require CD28 costimulation [17, 18]. Once T cells are fully activated, the CD3ζ signal is sufficient to trigger antigen-specific cytolysis independently of CD28 costimulation. This is reflected by the fact that pre-activated T cells execute cytolysis when stimulated via the physiological TCR/CD3 complex or via a recombinant immunoreceptor that harbors the CD3ζ signaling domain only.
By recording the T cell effector functions in detail, we elucidated [19] that CD28 costimulation obviously co-modulates the individual effector functions in different extent. This is obviously a general physiological phenomenon and not restricted to recombinant immunoreceptors.
CD28 signaling is not required but can enhance immunoreceptor triggered cytolytic activities;
CD28 costimulation is not required for but substantially increases IFN-γ secretion and T cell proliferation; and
CD28 costimulation is indispensable for induction of IL-2 secretion.
Tumor cells can induce antigen-specific tolerance or anergy on the basis of MHC class I-restricted antigen presentation and simultaneous lack of costimulatory ligands. Current immunotherapeutic strategies therefore aim to provide appropriate costimulatory signals on effector T cells to avoid induction of anergy.
Although cytolysis of tumor cells by redirected T cells is primarily independent of costimulatory signals, the efficiency of the T cell attack will be highly variable. This is likely to due to the variable expression of CD28 ligands on tumor cells since secondary functions that amplify the T cell response, like IL-2 secretion and T cell proliferation, depend on CD28 costimulation. IL-2 is moreover assumed to attract a second wave of antigen-unspecific inflammatory cells, e.g., natural killer cells, thereby locally enhancing the anti-tumor effect. Taken together, the anti-tumor reactivity in toto is thought to benefit from CD28 costimulation.
CD28 costimulation can be partially substituted by other costimulatory and adhesion molecules that, in contrast to CD28, are not restricted to APCs in their expression. For instance, ICAM-1 (CD54) substantially comodulates target cell lysis mediated by a recombinant scFv-γ chain immunoreceptor [20]. Correspondingly, ICAM-1 signaling without CD28 costimulation results in the induction of IL-2 secretion, although at low quantities [19]. This example indicates that apart from CD28 costimulation other molecules expressed on the target cell substantially modulate the anti-tumor response in a quantitative as well as qualitative fashion. As a consequence, T cells redirected by immunoreceptors are co-modulated in a certain, but not always predictable extent by costimulatory molecules expressed on tumor cells. To modulate immunoreceptor triggered T cell activation more precisely and independently of the costimulatory ligand on the surface of tumor cells, the costimulatory signal needs to be simultaneously present with the CD3ζ signal upon antigen engagement. This requirement resulted in the development of recombinant immunoreceptors with combined CD3ζ and CD28 signaling domains.
Immunoreceptors with dual signaling properties
Based on the impact of CD28 costimulation for immunotherapy, several groups aimed to deliver the CD28 signal in addition to the primary immunoreceptor signal. Alvarez-Vallina and Hawkins [21] generated a T cell line that simultaneously expresses two recombinant immunoreceptors, one with the CD3ζ and the other with the CD28 signaling domain, resulting in antigen triggered cellular activation and IL-2 secretion. To avoid coexpression of two different immunoreceptor molecules, Finney et al. [22] generated a recombinant receptor molecule that harbors both the CD3ζ and CD28 signaling domain integrated into one polypeptide chain resulting in enhanced cellular activation compared to signaling through CD3ζ alone. A similar receptor with dual signaling properties was subsequently described by our and other groups [22-26]. The design of the dual signaling immunoreceptor is based on its modular composition. Although the requirements for the individual receptor modules for stable receptor expression on the cell surface are yet not fully understood, functional analyses clearly demonstrate that costimulatory signals can be combined with the primary CD3ζ signal into a dual signaling receptor to modulate T cell effector functions in a predictive fashion. This is recently confirmed by knock-out of the functional domains of CD28 within the dual signaling receptor [27].
Stimulation of pre-activated T cells via the combined CD3ζ-CD28 immunoreceptor results in increased IFN-γ secretion and in secretion of substantial amounts of IL-2 whereas T cells equipped with the CD3ζ signaling receptor secrete lower amounts of IFN-γ and do not secrete IL-2. This process does not require exogenous B7/CD28 costimulation [26]. The specific cytolytic capacity, noteworthy, is not dramatically altered when T cells are stimulated via the CD28-CD3ζ dual signaling compared to the CD3ζ immunoreceptor. This is in accordance to previous observations that T cells stimulated via CD3ζ immunoreceptor eliminate target cells with B7 expression equally efficiently as target cells without B7 [26]. CD28 signaling moieties used so far in combined signaling immunoreceptors contain both the proliferation inducing and the apoptosis preventing domains. Utilizing these domains separately and combined with CD3ζ signaling, either proliferation or survival of activated T cells is thought to be sustained.
Other signaling domains, such as those of p56lck and CD4, have been combined with CD3ζ signaling also, resulting in improved receptor-mediated activation. Particularly, CD4-CD3ζ signaling, CD3ζ-lck and CD28-CD3ζ-lck signaling enhanced ZAP70 phosphorylation and IL-2 secretion and decreased the signaling threshold of receptor grafted T cells [28]. Because these combined signaling immunoreceptors have been analyzed so far only in established T cell lines, their impact on primary T cells with respect to antigen-triggered cytolysis, proliferation and cytokine secretion has still to be investigated.
Improved T cell proliferation and survival: other costimuli required?
Given the fact that immunoreceptor triggered T cell activation can be modulated by simultaneous CD28 costimulation, interest arises in combining other members of the CD28 family with CD3ζ into a costimulatory immunoreceptor molecule to provide appropriate costimulation upon antigen binding. The CD28 family includes in addition the cytotoxic-T-lymphocyte-antigen-4 (CTLA-4, CD152), inducible-costimulator (ICOS), OX40 (CD134), CD40-ligand (CD40L, CD154) and programmed-death-1 (PD-1). Although there is some functional overlap, each member of the CD28 family has distinct functions, depending on the nature of the stimuli and the antigenic history of the lymphocytes. ICOS and OX40 as well as 4-1BB (CD137) are expressed after CD28 signaling and are thought to be involved in prolonging the immune response and in generating T cell memory. In late T cell activation, OX40-OX40L interactions prolong IL-2 secretion and trigger the generation of memory T cells. 4-1BB signaling increases TCR-induced proliferation, survival, and cytokine production as well as CTL generation and tumor rejection.
We therefore generated a panel of combined signaling immunoreceptors that harbor either CD28, OX40 or 4-1BB as costimulatory domain, each in series with CD3ζ, and expressed these combined signaling receptors in pre-activated human T cells upon retroviral gene transfer. Recording the T cell effector functions in a comparative setting revealed that immunoreceptor triggered proliferation is dramatically increased by CD28, but not by OX40 or 4-1BB costimulation (Table 1). This is observed for both CD4+ and CD8+ T cells. Noteworthy, IL-2 secretion is only induced upon CD28, but not upon OX40 or 4-1BB costimulation whereas IFN-γ secretion is increased by each CD28, OX40 and 4-1BB costimulation when triggered simultaneously to CD3ζ signaling. The cytolytic activity, however, is not significantly altered by OX40 or 4-1BB costimulation compared to a moderate improvement upon CD28 costimulation. In CD4+ T cells, AICD is diminished upon costimulation compared to CD3ζ signaling only whereas in CD8+ T cells 4-1BB costimulation, but not CD28 and OX40 costimulation, prevents AICD. Taken together, each costimulus modulates a distinct pattern of T cell effector functions in its own fashion although there is some overlap. In resting T cells, CD28, ICOS, and OX40, each combined in series with CD3ζ into an immunoreceptor, enhanced receptor triggered, antigen specific cytolytic activities and ICOS, OX40, and 4-1BB, respectively, confers self-sufficient clonal expansion upon antigen encounter and enhanced cytokine secretion [29].
Table 1.
Summary of comparative analyses of T cell functions triggered by dual signaling immunoreceptors
| Proliferation | Cytokine secretion | Cytolysis | Protection from AICD | |||
|---|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-10 | ||||
| CD4+ T cell | ||||||
| CD3ζ | + | + | - | + | ++ | + |
| CD28-CD3ζ | +++ | +++ | ++ | ++ | +++ | ++ |
| CD3ζ-OX40 | + | +++ | - | (+) | ++ | +++ |
| 4-1BB-CD3ζ | + | +++ | - | (+) | ++ | +++ |
| CD8+ T cell | ||||||
| CD3ζ | + | + | - | + | ++ | + |
| CD28-CD3ζ | +++ | +++ | ++ | ++ | +++ | + |
| CD3ζ-OX40 | + | +++ | - | (+) | ++ | + |
| 4-1BB-CD3ζ | ++ | +++ | - | (+) | ++ | +++ |
Isolated human CD4+ and CD8+ T cells from the peripheral blood were equipped with an anti-CEA immunoreceptor with CD3ζ signaling domain only or with dual signaling domains by retroviral gene transfer, coincubated with solid phase bound antigen and subjected to functional analyses of proliferation by BrdU incorporation, cytokine secretion by ELISAs, and activation induced cell death (AICD) by flow cytometry. Specific cytotoxicity was recorded by an XTT-based viability assay upon coincubation with antigen expressing tumor cells. Coincubation with antigen-negative tumor cells and T cells without transduced immunoreceptor served as controls
Will triple-signaling receptors be superior?
Data currently available indicate that the CD3ζ signaling domain can be combined individually with the CD28, OX40, and 4-1BB costimulatory domain into one polypeptide chain in order to specifically modulate T cell effector functions. Since 4-1BB costimulation dramatically prevents AICD and, on the other hand, CD28 sustains T cell proliferation and cytokine secretion, including IL-2, combination of both the CD28 and 4-1BB domain with the CD3ζ signaling domain may be advantageous in order to sustain T cell survival and proliferation in vivo. Accordingly, Michel Sadelain and coworkers most recently reported that combined CD28 and 4-1BB signaling together with CD3ζ is superior in sustaining persistence of redirected T cells in vivo [30]. On the other hand, one-polypeptide chain immunoreceptors with triple signaling domains are at risk to recruit inefficiently the signaling molecules that trigger the downstream phosphorylation cascades. As a consequence, triple-signaling receptors may be unstable on the surface of T cells. Taken this into account, it remains to be elucidated whether triple signaling immunoreceptors in general will be superior in triggering persistence and function of redirected T cells.
Concluding remarks
It is generally assumed that costimulatory signals offer advantages for antigen-specific CTLs over strategies relying exclusively on TCR/CD3ζ triggering. The major advantage is that costimulation suppresses inhibitory and strengthens activating signals thereby tuning T cell effector functions. Different types of costimulation result in different patterns of activation profiles. The individual costimulatory pathways are therefore worth to be explored in more detail with respect for use in the immunoreceptor strategy. For instance, the inhibitory effect of TGF-β on redirected T cell proliferation may be counteracted by CD28 costimulation (Koehler, Kofler, Hombach, Abken, unpublished). The multiple pathways by which non-tolerized T cells can be stimulated to inhibit their entry into a tolerant state are moreover potential candidates to be integrated into dual or triple signaling immunoreceptors. The combinatory use of OX40 and 4-1BB signaling may represent a promising approach to break immune tolerance and to increase T cell survival and proliferation in order to strengthen the anti-tumor response. CD4+ T cells in particular will be valuable targets to revert tolerance and to induce a long-lasting, tumor-specific memory since engagement of OX40 during tumor priming enhances CD4+ T cell memory for tumor antigens [31].
Costimulation through B cell-activating factor (BAFF) is potentially advantageous for tumor-specific T cells that are not sufficiently activated due to low antigen expression levels on tumor cells. This assumption is based on the fact that BAFF induces proliferation of T cells that are suboptimally stimulated through their TCR [32]. CD58-CD2 costimulation may alternatively be suitable to increase the overall level of TCR signaling since CD58-CD2 costimulation of CD4+ memory T cells does not trigger a single distinct costimulatory pathway, but rather amplifies several pathways downstream of the TCR [33].
The success of the immunoreceptor strategy will require the fulfillment of several criteria in addition to appropriate costimulation, including low intra- and inter-malignant lesion heterogeneity, the effective penetration of the malignant lesions, the persistence of tumor-specific T cells, prevention of AICD or the functional resistance towards regulatory T cells. Abnormalities in the signal transduction pathways, however, will render costimulation ineffective. Since T cells in patients with advanced stages of the disease are frequently defective in TCR signaling, the immunoreceptor approach will require repeated administration of “freshly-grafted T cells to substitute burn-out CTLs and cells that may be anergized. The disadvantage of repeated administration may be an advantage at the same time to provide repeatedly new waves of a cellular immune attack. Independently of the intrinsic signaling defects of T cells, costimulation-independent tolerance mechanisms, such as HLA-G, IL-10 and TGF-β, will impair T cell activation severely.
Redirected T cells with new specificities when adoptively transferred harbor the risk of initiating auto-immune reactions based on cross-reactivities with healthy tissues. This was observed in a most recent clinical trial [34]. Decreasing the threshold for activation by appropriate costimulation moreover increases the risk to initiate unwanted T cell activation resulting in auto-immunity. The trial, on the other hand, clearly demonstrated that the adoptively transferred T cells can rapidly be eliminated by steroids thereby limiting the auto-immune reactions.
T cell homeostasis seems to be of major importance for the expansion and survival of adoptively transferred T cells. It may therefore be of benefit to transfer engineered T cells into lymphodepleted hosts. Clinical trials that are implemented in the near future will address this issue in order to increase the in vivo efficacy of a redirected T cell attack.
Despite a number of unresolved questions, modulation of costimulation combined with sensitization to antigen provides an attractive strategy to provide an effective tumor-specific T cell response. Clinical trials will determine whether tuning antigen-specific T cell activation by costimulation can strengthen the anti-tumor immune attack without increasing the risk of aggressive auto-immunity.
Acknowledgments
Our work was supported by the Deutsche Forschungsgemeinschaft, Deutsche Krebshilfe, Wilhelm Sander-Stiftung, the European Commission through the ATTACK consortium and the Köln Fortune Program of the University of Cologne.
Footnotes
This article is a symposium paper from the conference “Cancer Immunotherapy 2006 Meets Strategies for Immune Therapy- held in Mainz, Germany, on 4- May 2006.
References
- 1.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. . Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 3.Schumacher TN. T-cell-receptor gene therapy. . Nat Rev Immunol. 2002;2:512–519. doi: 10.1038/nri841. [DOI] [PubMed] [Google Scholar]
- 4.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Opalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A., de Vries CR, Rogers-Freezer LJ, Maroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross G, Gorochov G, Waks T, Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. . Transplant Proc. 1989;21:127–130. [PubMed] [Google Scholar]
- 6.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. . Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abken H, Hombach A, Heuser C. Immune response manipulation: recombinant immunoreceptors endow T-cells with predefined specificity. . Curr Pharm Des. 2003;9:1992–2001. doi: 10.2174/1381612033454289. [DOI] [PubMed] [Google Scholar]
- 8.Hombach A, Heuser C, Abken H. The recombinant T cell receptor strategy: insights into structure and function of recombinant immunoreceptors on the way towards an optimal receptor design for cellular immunotherapy. . Curr Gene Ther. 2002;2:211–226. doi: 10.2174/1566523024605573. [DOI] [PubMed] [Google Scholar]
- 9.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Kruis W, Pohl C, Abken H. T cell targeting of TAG72 + tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. . Gastroenterology. 1997;113:1163–1170. doi: 10.1053/gast.1997.v113.pm9322511. [DOI] [PubMed] [Google Scholar]
- 10.Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, Smyth FE, Cartwright GA, Power BE, Honemann D, Prince HM, Darcy PK, Kershaw MH. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. . Proc Natl Acad Sci USA. 2005;102:19051–19056. doi: 10.1073/pnas.0504312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, Groner B. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. . Clin Cancer Res. 1996;2:1001–1008. [PubMed] [Google Scholar]
- 12.Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, Kershaw MH, Smyth MJDarcy PK. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. . J Immunol. 2002;169:5780–5786. doi: 10.4049/jimmunol.169.10.5780. [DOI] [PubMed] [Google Scholar]
- 13.Hombach A, Schlimper C, Sievers E, Frank S, Schild HH, Sauerbruch T, Schmidt-Wolf IG, Abken H. A recombinant anti-carcinoembryonic antigen immunoreceptor with combined CD3ζ-CD28 signalling targets T cells from colorectal cancer patients against their tumour cells. . Gut. 2006;55:1156–1164. doi: 10.1136/gut.2005.076208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hombach A, Muche JM, Gerken M, Gellrich S, Heuser C, Pohl C, Sterry W, Abken H. T cells engrafted with a recombinant anti-CD30 receptor target autologous CD30+ cutaneous lymphoma cells. . Gene Ther. 2001;8:891–895. doi: 10.1038/sj.gt.3301467. [DOI] [PubMed] [Google Scholar]
- 15.Hombach A, Heuser C, Marquardt T, Wieczarkowiecz A, Groneck V, Pohl C, Abken H. CD4+ T cells engrafted with a recombinant immunoreceptor efficiently lyse target cells in a MHC antigen- and Fas-independent fashion. . J Immunol. 2001;167:1090–1096. doi: 10.4049/jimmunol.167.2.1090. [DOI] [PubMed] [Google Scholar]
- 16.Hombach A, Köhler H, Rappl G, Abken H. Human CD4+ T cells lyse tumor cells via granzyme/perforin upon circumvention of MHC restriction by grafting with a recombinant, antibody-derived immunoreceptor. J Immunol. 2006;177:5668–675. doi: 10.4049/jimmunol.177.8.5668. [DOI] [PubMed] [Google Scholar]
- 17.Friedmann-Morvinski D, Bendavid A, Waks T, Schindler D, Eshhar Z. Redirected primary T cells harboring a chimeric receptor require costimulation for their antigen-specific activation. . Blood. 2005;105:3087–3093. doi: 10.1182/blood-2004-09-3737. [DOI] [PubMed] [Google Scholar]
- 18.Brocker T, Riedinger M, Karjalainen K. Redirecting the complete T cell receptor/CD3 signaling machinery towards native antigen via modified T cell receptor. . Eur J Immunol. 1996;26:1770–1774. doi: 10.1002/eji.1830260816. [DOI] [PubMed] [Google Scholar]
- 19.Hombach A, Sent D, Schneider C, Heuser C, Koch D, Pohl C, Seliger B, Abken H. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. . Cancer Res. 2001;61:1976–1982. [PubMed] [Google Scholar]
- 20.Weijtens ME, Willemsen RA, van Krimpen BA, Bolhuis RL. Chimeric scFv/gamma receptor-mediated T-cell lysis of tumor cells is coregulated by adhesion and accessory molecules. . Int J Cancer. 1998;77:181–187. doi: 10.1002/(SICI)1097-0215(19980717)77:2<181::AID-IJC2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. . Eur J Immunol. 1996;26:2304–2309. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- 22.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. . J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 23.Eshhar Z, Waks T, Bendavid A, Schindler DG. Functional expression of chimeric receptor genes in human T cells. . J Immunol Methods. 2001;248:67–76. doi: 10.1016/S0022-1759(00)00343-4. [DOI] [PubMed] [Google Scholar]
- 24.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. . Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 25.Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, Kershaw MH, Smyth MJ, Darcy PK. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. . Blood. 2002;100:3155–3163. doi: 10.1182/blood-2002-04-1041. [DOI] [PubMed] [Google Scholar]
- 26.Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, Seliger B, Abken H. Tumor specific T cell activation by recombinant immunoreceptors: CD3zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 27.Moeller M, Haynes NM, Trapani JA, Teng MW, Jackson JT, Tanner JE, Cerutti L, Jane SM, Kershaw MH, Smyth MJ, Darcy PK. A functional role for CD28 costimulation in tumor recognition by single-chain receptor-modified T cells. . Cancer Gene Ther. 2004;11:371–379. doi: 10.1038/sj.cgt.7700710. [DOI] [PubMed] [Google Scholar]
- 28.Geiger TL, Nguyen P, Leitenberg D, Flavell RA. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in lymphocytes. Blood. 2001;98:2364–2371. doi: 10.1182/blood.V98.8.2364. [DOI] [PubMed] [Google Scholar]
- 29.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. . J Immunol. 2004;172:104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 30.Zhong XS, Matsushita M, Saudemont A, Santos E, Sadelain M. Integrated CD28 and 4-1BB signals strongly potentiate CD8+ T cell mediated eradication of metastatic prostate cancer. Mol Ther. 2006;13(Suppl1):S103. doi: 10.1016/j.ymthe.2006.08.323. [DOI] [Google Scholar]
- 31.Weinberg AD. OX40: targeted immunotherapy–implications for tempering autoimmunity and enhancing vaccines. . Trends Immunol. 2002;23:102–109. doi: 10.1016/S1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 32.Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell costimulation by the TNF ligand BAFF. . J Immunol. 2001;167:6225–6231. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 33.Mestas J, Hughes CC. Endothelial cell costimulation of T cell activation through CD58-CD2 interactions involves lipid raft aggregation. . J Immunol. 2001;167:4378–4385. doi: 10.4049/jimmunol.167.8.4378. [DOI] [PubMed] [Google Scholar]
- 34.Lamers CH, Sleijfer S., Vulto AG, Kruit WH, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]