Abstract
HLA multimers are now widely used to stain and sort CD8 T lymphocytes specific for epitopes from viral or tumoral antigens presented in an HLA class I context. However, the transfer of this technology to a clinical setting to obtain clinical grade CD8 T lymphocytes that may be used in adoptive cell transfer (ACT) is hindered by two main obstacles: the first obstacle is the use of streptavidin or derived products that are not available in clinical grade to multimerize HLA/peptide monomers and the second is the reported high degree of apoptosis that eventually occurs when T cell receptors are crosslinked by HLA multimers. In the present report, we describe new HLA multimers composed of immunomagnetic beads covalently coupled to a mAb specific for the AviTag peptide and coated with HLA/peptide monomers bearing the non biotinylated AviTag at the COOH terminus of the HLA heavy chain. Thus, all the components of this new reagent can be obtained in clinical grade. We compared these new multimers with the previously described multimers made with streptavidin beads coated with biotinylated HLA/peptide monomers, in terms of sorting efficiency, recovery of functional T cells, apoptosis and activation. We provide evidence that the new multimers could very efficiently sort pure populations of T lymphocytes specific for three different melanoma antigens (Melan-A, gp100 and NA17-A) after a single peptide stimulation of melanoma patients’ PBMC. The recovered specific T cells were cytotoxic against the relevant melanoma cell-lines and, in most cases, produced cytokines. In addition, in marked contrast with streptavidin-based multimers, our new multimers induced very little apoptosis or activation after binding specific T lymphocytes. Altogether, these new multimers fulfill all the necessary requirements to select clinical grade T lymphocytes and should facilitate the development of ACT protocols in cancer patients.
Keywords: Immunotherapy, Cell-sorting, HLA multimers, Melanoma, T lymphocytes
Introduction
In immunotherapy, two main strategies are developed to exploit the therapeutic potential of specific T cells: active immunotherapy aims at inducing specific responses (acute and memory T cell responses) of the host immune system by vaccination with antigens (viral or tumoral) and passive immunotherapy relies on the adoptive transfer of reactive T lymphocytes produced ex-vivo. An advantage of adoptive cell therapy (ACT) over vaccination is that (1) it allows an accurate control of quality, quantity and functionality of the effector cells to be re-injected and (2) it may bypass tolerance induction mechanisms that take place in vivo. In viral immunity, a number of reports have already demonstrated the efficiency of the infusion of T cell populations enriched in specific T cells: for example, infusion of anti-EBV (Epstein–Barr virus) T cells has been shown to prevent EBV-associated lympho-proliferative diseases after hematopoietic stem cell and solid organ transplantation [1–3].
In cancer, the best argument in favor of ACT is the demonstration that it can elicit clinical regressions of cancers not curable by other treatments. Initially established for hematopioietic tumors in an allogeneic setting [4], the beneficial effect of ACT has also been documented in autologous situations such as the control of EBV-induced tumors by virus-antigen specific T cells [1]. Later on, we and others have demonstrated the clinical benefit of ACT in melanoma treatment by showing a correlation between tumor reactive TIL (Tumor Infiltrating Lymphocytes) transfer and high rate of tumor regressions or, in an adjuvant setting, melanoma relapse prevention [5–7]. Our study suggested that tumor-reactive TIL transfer may be an efficient treatment in melanoma when performed at an early stage of the disease. We reckon that the efficacy of ACT may be improved by injecting pure tumor-reactive T cells instead of non selected TIL and, for that purpose, we aim at developing a reliable procedure to sort and expand T lymphocytes specific for selected tumor antigens. One way of obtaining such pure T lymphocyte populations is to select specific T cells through their TCR using HLA multimers loaded with dominant epitopes from tumor antigens although there has been concerns about the induction of apoptosis by such TCR crosslinking [8, 9]. Despite the possible apoptotic death of some specific T cells during or following the sort, multimer-selected T lymphocytes have been proven efficient to control CMV infection after stem cell transplantation [10]. Such a strategy remains to be evaluated in cancer. It should be underlined however that the procedure used by Cobbold et al. (i.e. staining of lymphocytes with non GMP PE-conjugated tetramers followed by a sort with anti-PE magnetic beads) did not meet the usual standards and regulations for clinical grade reagents. Likewise, the sorting procedure based on biotinylated HLA/peptide monomers coated on streptavidin immunomagnetic beads that we initially used in viral immunity [11] and recently applied to the selection of melanoma-reactive T cells [12] cannot be approved for clinical use because of its use of streptavidin. We have thus designed new HLA/peptide multimers using M450 immunomagnetic beads covalently coupled to a monoclonal antibody against the AviTag™ peptide and coated with HLA/peptide complexes so that all components can be made in clinical grade. In the present report, we compared those new multimers with the previous ones in terms of (1) sorting efficiency of specific T cells using different melanoma antigens (2) induction of AICD (activation induced cell death) (3) induction of cytokine production. We demonstrate that the new procedure is at least as efficient as the previous one but presents the additional benefit of inducing very little apoptosis and activation, thus minimizing the risk of losing reactive clonotypes or functionally altering the selected T cells.
Materials and methods
Cell lines
Melanoma cell lines (“M”) were established in our laboratory from metastatic tumor fragments as previously described [13]. The TAP-deficient human hybrid cell line CEM × 721 T2 (“T2”) used as presenting cell was a gift from T. Boon (Ludwig Institute for Cancer Research, Brussels, Belgium). The EBV-B-transformed cell line LAZ 338 (“LAZ”) was a gift from T. Hercend (Vertex Pharmaceutical, Abingdon, UK).
Melanoma TIL
Short-term cultured TIL (from the unit of cellular and gene therapy, Pr Dreno, Nantes) were isolated by culturing cryopreserved fragments of stage III metastatic lymph nodes into 12-well tissue culture plates with X-Vivo 15 serum-free medium (Bio*Whittaker, Walkersville, MD, USA) containing rIL2 (150 UI/ml) (Eurocetus, Reuil-Malmaison, France) and l-glutamine (1 nM) for 10–14 days and then expanded on feeder cells as previously described [14].
PBMC, T cell-lines and clones
Blood from HLA-A2 melanoma patients donors (Unit of Skin Cancer, Centre Hospitalier Régional Hotel Dieu, Nantes) was collected following approval of the study by the local Institutional Ethics Committee and written informed consent from each patient. After blood collection (30 ml), human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient centrifugation and washed with RPMI 1640 medium. PBMC were stimulated for 14 days with 10 μM of the Melan-A26-35 A27L analogue [15] or the gp100280–288 [16] or the NA17-A peptide [17], in RPMI 1640 medium containing 8% human serum (culture medium) supplemented with 50 Uml IL-2 (Chiron, clinical grade), l-glutamine (2 nM, Invitrogen Life Technologies), penicillin (100 UI/ml) and streptomycin (100 µg/ml). Peptides used were purchased from Eurogentech (Brussels, Belgium) and their purity, controlled by reversed-phase HPLC, was above 80%. Lyophilized peptides were dissolved in DMSO at 10 mg/ml and stored at −20°C before use. The two Melan-A/A2 specific T cell-lines used in the study of apoptosis were obtained by sorting of peptide-stimulated PBMC with biotinylated HLA/peptide complexes coated on streptavidin beads as described in a previous publication [12]. The Melan-A specific T cell clones obtained by cloning of peptide-stimulated PBMC have been previously described [18].
Construction of HLA-A*0201/peptide tetramers
HLA-A*0201/peptide α3-mutated monomers were generated as previously described [11] except that the original biotinylation sequence (LHHILDAQ KMVWNHR) was replaced by the AviTag sequence (GLNDIFEAQKIEWHE) (Avidity, Aurora, CO, USA). Recombinant proteins were produced as inclusion bodies in Escherichia coli XA90’Lacq1, dissoved in 8 M urea, and refolded with 50 µg/ml of Melan-A/MART1-A26–35 A27L peptide (ELAGIGILTV), gpP100280–288 peptide (YLEPGPVTA) or NA17-A peptide (VLPDVFIRC) purchased from Eurogentech (Brussels, Belgium). Tetramerisation was performed as previously described. Briefly, HLA monomers were biotinylated for 4 h at 30°C with 6 μg/ml BirA (Immunotech), purified on monoQ column (Pharmacia, St Quentin en Yveline, France) and tetramerised with PE or APC-labeled streptavidin (Beckton Dickinson, Grenoble, France) at a molar ratio of 4:1.
Production of the anti-Avitag™ mAb, AvT-6A8
The aim was to select a high affinity monoclonal antibody that recognizes the peptide MSGLNDIFEAQKIEWHE, named AviTag™ (Avidity). To this end, we added a cystein residue at the NH2 end of the AviTag peptide to chemically couple this peptide to KLH (Keyhole Limpet Hemocyanin) with the MBS (Maleimido benzoyl N hydroxy succinidyl ester) agent. This Avitag-KLH was used to immunize Balb/c mice. Splenocytes were fused with murine myeloma cells SP2/0-AG-14 and hybridomas were screened by ELISA using the AviTag™ coupled to ovalbumine (OVA) with SMCC (Succinimidyl 4-[N-maleimidomethyl] cyclohexane-1-carboxylate). The hybridoma 6A8 was chosen because of its highest reactivity towards the peptide AviTag and sub-cloned. The monoclonal antibody named AvT-6A8 used in this study was purified from hybridoma supernatant using a protein A column.
Surface plasmon resonance analysis
Binding experiments of the anti-AviTag AvT 6A8 antibody to a HLA-A*0201/Melan-A A27L soluble complex were performed with a BIAcore 2000 optical biosensor (BIAcore, GE Healthcare, Orsay, France). The mAb AvT 6A8 was covalently coupled to a carboxymethyl dextran flow cell (CM5 BIAcore) as recommended by the manufacturer. (The level of immobilization was set at 500 resonance units.) Binding of HLA-A*0201/Melan-A A27L was measured at 25°C with concentrations ranging from 0.625 to 10 nM at a flow rate of 40 μl/min in HBS-EP buffer (0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% surfactant P20). Association was monitored for 5 min before initiating the dissociation phase for another 10 min with HBS-EP buffer. Flow cells were regenerated by a 1-min pulse with 10 mM glycine–HCl at pH 1.8. The resulting sensorgrams were analyzed using the BIA Evaluation software 3.2.
Preparation of immunomagnetic beads and cell sorting
Optimal conditions for coating M450 beads (Dynabeads M-450-epoxy, Dynal, Compiegne, France) were determined by incubating 5 × 107 beads at room temperature with increasing amounts of anti-AviTag 6A8 antibody (25–150 μg) in borate buffer for various times. After two washes with PBS/0,1% BSA, the quantity of mAb bound to the beads was assessed by measuring the mean fluorescence intensity after staining with a goat anti mouse PE-conjugated F(ab′)2 (Beckman-Coulter, Marseille, France). Maximal binding was achieved after a 1 h incubation with 50 μg of AvT-6A8 mAb. Similarly, coating of HLA-A2/peptide complexes onto M280 streptavidin or M450-AvT-6A8 was tested by incubating 5 × 106 beads for 1 h at room temperature with various range of biotinylated or unbiotinylated HLA-A*0201/peptide complexes, respectively. Quantity of HLA/peptide complexes coated onto the two types of beads was assessed by the mean fluorescence intensity analysed by flow cytometry after staining with a FITC-conjugated antibody against HLA-ABC (Beckman-Coulter, Marseille, France). For M450-AvT beads, maximal coating was achieved with a concentration of 10 μg/ml of HLA/peptide complexes. For M280 streptavidin beads, saturation was obtained with 2 μg/ml of HLA/peptide monomers (noted M280s for saturated). Coating with 0.03 μg/ml gave us a quantity of complexes onto M280 beads (noted M280us1 for unsaturated 1) corresponding to the amount of complexes on saturated M450-AvT while coating with 0.0075 μg resulted in 2.5 times less complexes then M450-AvT (M280us2) corresponding approximatively to the density of complexes on M450-AvT. PBMC or TIL were mixed with monomer-coated beads (M280us or M450-AvT) (ratio 1:1) and rotated for 4 h at 4°C in 1 ml of PBS/0.1% BSA. At the end of the incubation, beads were washed ten times as follows: beads were magnetized with Dynal magnet for 1 min, supernatant was removed and beads were re-suspended in PBS/0.1%. At the end of washes, cells coated onto the beads were expanded using polyclonal T cell stimulation. M450-AvT beads were stable for two months at 4°C and were coated extemporaneously with HLA/peptide monomers for each experiment. The amounts of HLA complexes bound to M450-AvT was very reproducible as assessed by flow cytometry with a mean fluorescence beween 30 and 40 in more then 30 preparations performed.
Chromium cytotoxicity assay
Cytotoxic activity was measured in a standard 4 h assay against 51Cr-labeled cells. Briefly, target cells (peptide pulsed T2 or melanoma cells) were incubated with 100 μCi Na2 51CrO4 at 37°C during 1 h. For peptide avidity assays, T2 cells were loaded with a range of peptide concentrations for 1 h at 37°C and washed. Effector cells were then added at various E:T ratio (10:1 for T2 cells and 2:1, 10:1 and 50:1 on melanoma cell-lines) in a final volume of 100 μl culture medium. After a 4 h co-culture, 25 μl of supernatant were collected and mixed with 100 μl of scintillation cocktail (Optiphase Supermix, Wallak, UK) for measurement of radioactivity content using a betaplate counter (Perkin Elmer, Lifescience Courtaboeuf, France).
Cytokine production assay
Specific lymphocytes (1 × 105) were stimulated by peptide-pulsed T2 cells (3 × 105) for 6 h at 37°C in 200 μl of culture medium in the presence of brefeldin A (10 μg/ml) (Sigma, St Louis, MO, USA) in round-bottom 96-well plates. For intracytoplasmic cytokine staining, cells were fixed for 10 min at room temperature in PBS 4% paraformaldehyde (SIGMA), washed and stored at 4°C until labelling. Fixed lymphocytes were stained for cytokine production using anti-IL2, anti-TNF-α and anti-IFN-γ specific antibodies (BD Biosciences, France) in PBS 0.1% BSA, 0.1% saponin. After staining, cells were resuspended in PBS and analyzed on a LSR flow cytometer using Cell Quest software (BD Biosciences).
TCR β-chain V region expression analysis
A panel of 24 PE or FITC coupled anti-Vβ Abs was used to evaluate the T cell repertoire (BCI, Marseille, France). Staining was performed in PBS, 0.1% BSA for 45 min at 4°C before analysis by flow cytometry.
Apoptosis assays
To evaluate the apoptosis induced by the different types of multimers, specific T cell clones or cell lines were incubated with an equal number of either M280s, M280us or M450-AvT in 96-well plates at 37°C in culture medium. The first method that we used to detect T cells undergoing apoptosis was a double staining with FITC annexin-V and propidium iodide. At the end of a 6 h incubation, 1 × 105 specific T cells were collected, washed twice with cold buffer and incubated 15 min with propidium iodide and annexin-V-FITC, according to the manufacturer’s protocol (Beckton Dickinson, Grenoble, France). Flow cytometry was performed on a FACS LSR using the Cell Quest software (BD). The second method to evaluate apoptosis was the Caspase-Glo assay. The caspase-Glo® 3/7 and 9 preparation (Promega, Charbonnières, France) lyophilized substrates (respectively DEVD and LEHD-aminoluciferin powder) were dissolved in caspase Glo lysis buffer as recommended by the manufacturer. Proteasome inhibitor MG132 was added to caspase glo 9® assay to reduce non-specific background. T cells were incubated with beads in 96-well plates for 4 h and then a sample of 1.8 × 104 T cells in 25 μl was mixed with an equal volume of Caspase Glo® reagent, and placed in the dark for 45 min. Luminescence was quantified with a betaplate counter (Perkin Elmer, Lifescience Courtaboeuf, France).
Statistical analysis
Data are expressed as mean ± SEM. Normal distribution of value were analysed using Kolmogorov–Smirnov test. Mean comparisons were performed by ANOVA with Bonferoni post test.
Results
Preparation of the new HLA/peptide multimers
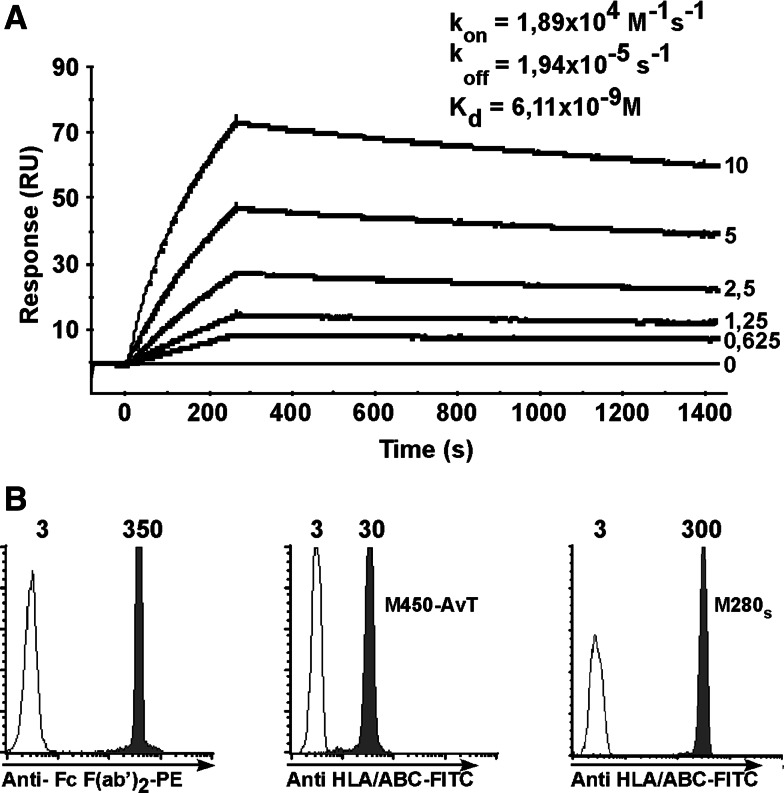
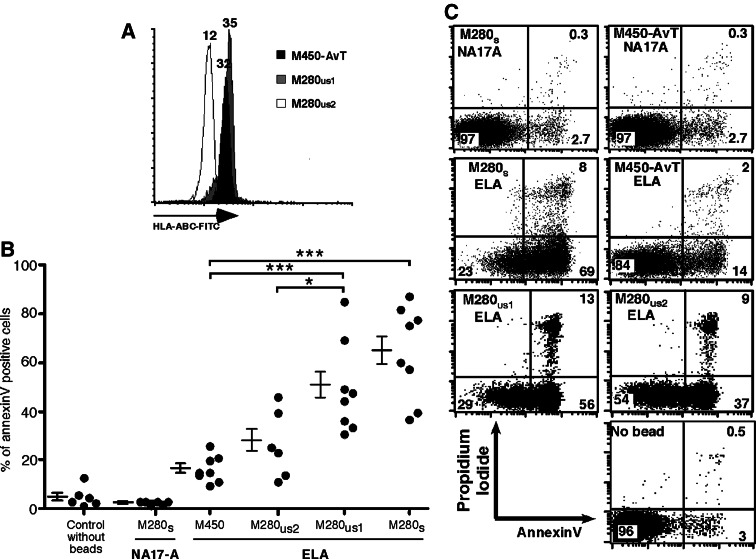
The initial purpose for designing a new HLA multimer-based sorting procedure was to avoid the use of streptavidine to coat HLA complexes onto magnetic beads because clinical grade streptavidin is not available. We thus decided to replace it by a monoclonal antibody, AvT-6A8 (patent pending), directed against the AviTag™ peptide (Avidity). The affinity of AvT-6A8 mAb for HLA complexes bearing the AviTag at the C terminal end of the HLA heavy chain was measured by plasmon surface resonance. AvT-6A8 mAb was covalently coupled to CM5 chips and the analyte was soluble HLA-A*0201 complexes loaded with the Melan-A A27L epitope. The sensorgrams are shown on Fig. 1a, the calculated Kd value of AvT-6A8 was 6.1 × 10−9 M which denotes a good affinity of the antibody for the monomers bearing the AviTag. We then covalently coupled mAb AvT-6A8 to M-450 epoxy magnetic beads (Invitrogen) and evaluated the quantity of coupling by flow cytometry after staining with a PE-labeled goat anti-mouse Fc F(ab)′2 mAb. As shown on Fig. 1b, left panel, AvT-6A8 was efficiently coupled to the beads. We then tested various incubation conditions of AvT-6A8-beads with soluble HLA-A2/Melan-A A27L to maximize binding of the HLA complexes onto the beads. Binding of HLA complexes was evaluated by staining with an FITC-conjugated anti-HLA-ABC mouse mAb. The maximal quantity of HLA complexes on the beads obtained after a 1 hour incubation at room temperature was rather low (MFI = 30) (Fig. 1b, middle panel) when compared to that obtained with saturating amounts of the same biotinylated HLA complexes coated onto M280-streptavidine beads (MFI = 300) (Fig. 1b, right panel). We concluded that only a small fraction of covalently coupled AvT-6A8 was able to bind the tagged HLA-A2/peptide complexes, probably due in part to steric hindrance.
Fig. 1.
a The mAb AvT-6A8 directed against the AviTag peptide was covalently coupled to a CM5 BIAcore chip and soluble HLA-A*0201/Melan-A-27L monomers (concentrations ranging from 0.625 to 10 μg/ml) were assayed for binding at 25°C. Shown are sensorgrams and calculated kinetic constants (average value from two independent experiments). b The mAb AvT 6A8 was covalently coupled to M450-epoxy beads and amounts of bound antibody was assessed by staining with a PE-conjugated goat anti-mouse-Fc F(ab′)2 (grey peak, left panel). Unbiotinylated and biotinylated HLA-A*0201/Melan-A-27L were coated on M450-AvT-6A8 and M280-streptavidin respectively. Amounts of HLA/peptide complexes coated on the beads were assessed by staining with a FITC-conjugated mouse anti-human HLA-ABC antibody (grey peak, middle and right panels). White histograms correspond to staining of uncoated beads
Comparison of sorting/amplification efficiencies using HLA multimers made with AvT-6A8-M450 beads vs streptavidin M280 beads
Despite the fact that M450 beads are bigger than M280 (diameter of 2.8 μm for M280 and 4.5 μm for M450), saturation of AvT-6A8-M450 beads (M450-AvT) led to a lower quantity of HLA complexes at their surface than the quantity achieved by saturation of previously validated M280 streptavidin beads (M280s) [11]. It was therefore important to compare the sorting efficiencies of the two types of beads. This was performed by sorting 4 populations from 3 different melanoma patients (3 Melan-A A27L-stimulated PBMC populations and 1 unstimulated TIL population) containing various percentages of HLA-A2/Melan-A specific T cells with the two types of multimers. PBMC stimulation was performed by simply adding the Melan-A A27L peptide at 10 μM to the cells and keeping them in culture for 15 days with 50 UI of IL2 (see Materials and methods).
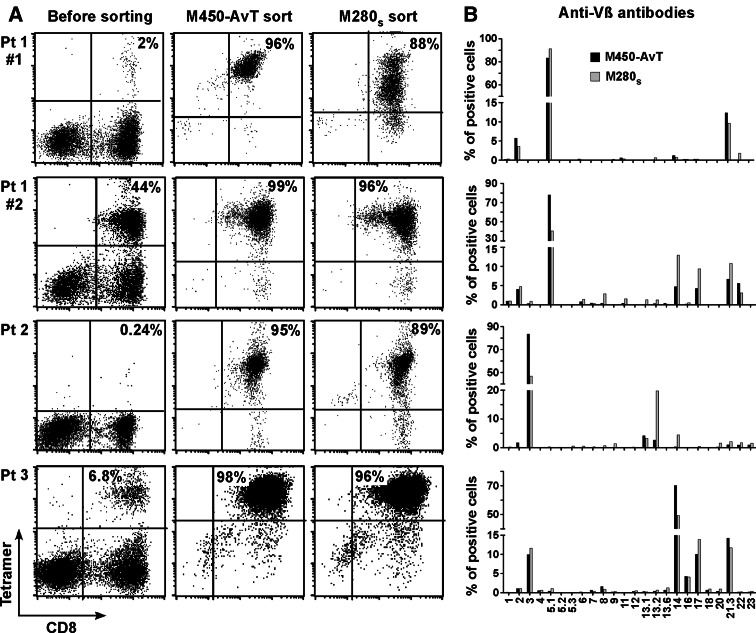
Figure 2a shows the percentage of CD8/tetramer positive lymphocytes before and after the sort with the two types of beads. It can be observed that, regardless of the percentage of specific cells (ranging from 0.24 to 44%) within the starting populations, multimers made with AvT-6A8-beads were at least as efficient, if not slightly more, as those made with streptavidin beads to select Melan-A specific T cell populations. Table 1 summarizes the data concerning these sorts: in all cases, a single sort of 4–5 × 106 PBMC performed with M450-AvT led to the obtention of almost pure Melan-A specific CD8 + T cells (above 95%) that greatly expanded during the 15 days of non specific post-sort restimulation (enrichment factor ranging from 422 to 4,563). Thus, in terms of efficiency and subsequent in vitro amplification, the data obtained with multimers made with M450-AvT did not significantly differ from those obtained with multimers made with M280s.
Fig. 2.
M450-AvT and M280s sort of Melan-A specific T cell and analysis of repertoire diversity. a PBMC or TIL from three melanoma patients were stimulated with the Melan-A26–35 A27L peptide for 14 days, sorted with HLA-A*0201/Melan-A-27L multimers made with either M450-AvT or M280s beads and restimulated non specifically for 14 days. Percentages of specific T cells was assessed by double staining with PE-conjugated tetramer and FITC-conjugated anti-CD8 antibody. As indicated, patient 1 was tested in two independent experiments. b Repertoire diversity of M450 (black bar) and M280 (grey bar) sorted populations was assessed by labeling with 24 anti-Vβ antibodies
Table 1.
Enrichment and amplification yields in Melan-A, GP100 and NA17-A specific T cells after MHC/peptide multimer sorting
| Melan-A | GP-100 | NA17-A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 #1 (PBMC) | Pt 1 #2 (PBMC) | Pt 2 (PBMC) | Pt 3 (TIL) | Pt 5 (PBMC) | Pt 6 (PBMC) | |||||
| Starting population | ||||||||||
| Total cell number (×106) | 4 | 4 | 5 | 4 | 10 | 2.8 | ||||
| CD8 specific cells (%)a | 2 | 44 | 0.24 | 6.8 | 0.23 | 0.1 | ||||
| CD8 specific cells nbb | 8 × 104 | 1.76 × 106 | 1.2 × 103 | 2.72 × 105 | 2.3 × 104 | 2.8 × 103 | ||||
| D14 after sort/amplification | ||||||||||
| Cell sorting condition | M450-AvT | M280s | M450-AvT | M280s | M450-AvT | M280s | M450-AvT | M280s | M450-AvT | M450-AvT |
| Amplified cell nb (×106) | 380 | 210 | 751 | 1,000 | 15.4 | 23.4 | 150 | 120 | 26 | 1.2 |
| CD8 specific cells (%)a | 96 | 88 | 99 | 96 | 95 | 89 | 98 | 96 | 90 | 95 |
| CD8 specific cells nbb | 3.65 × 108 | 1.85 × 108 | 7.43 × 108 | 1.1 × 109 | 1.42 × 107 | 1.78 × 107 | 1.47 × 108 | 1.15 × 108 | 2.34 × 107 | 1.08 × 106 |
| Enrichment factorc | 4,563 | 2,313 | 422 | 625 | 1,183 | 1,483 | 529 | 415 | 1,017 | 386 |
aPercentage of T cells specific for HLA*A201/peptide complexes is determined by CD8/tetramer double labeling
bNumber of specific cells (percentage of tetramer labeled cells multiplied by the total number of cells)
cEnrichment factor is the ratio between the number of specific cells after and before one sort/amplification cycle
We then asked whether multimers made with M450-AvT selected the same T cell repertoire against HLA-A2/Melan-A than multimers made with M280s and thus compared Vβ usage within populations sorted with the two types of beads (Fig. 2b). In each of the three patients analyzed, our panel of antibodies allowed the detection of more than 95% of the reactive repertoire. We observed no significant difference between the two sorted populations in terms of Vβ usage i.e. the major Vβ subpopulations were present in both populations in all cases even if quantitative differences could be observed within Vβ sub-families. In the case of patient 1, from whom two different culture wells were tested (Pt 1 #1 and Pt 1 #2), it can be noted that the degree of polyclonality of the sorted population increased with the percentage of specific cells within the starting population and this was seen with both types of beads. Thus M450-AvT multimers, although presenting a lower density of HLA complexes, did not sort a more restricted T cell repertoire against Melan-A/A2 than the M280s multimers.
Functional analyses of T lymphocytes sorted with the two types of beads
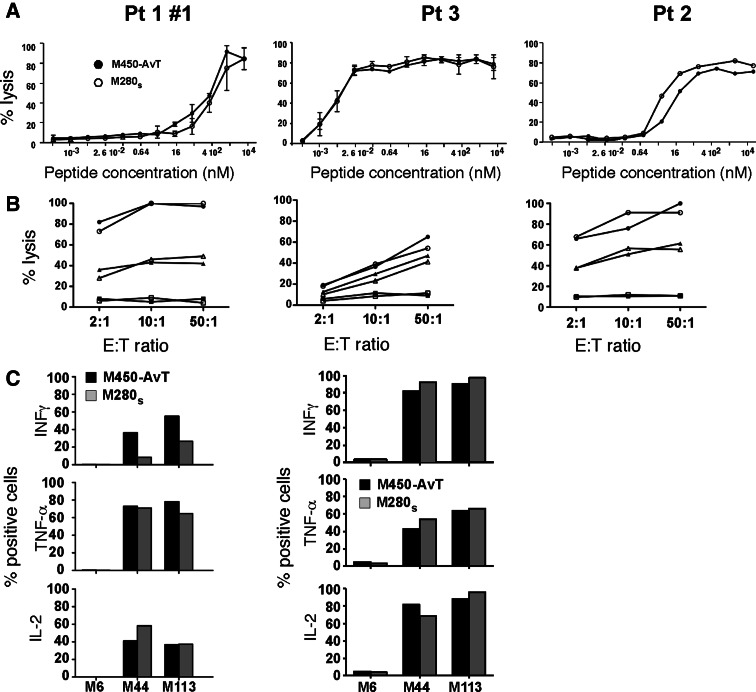
We next compared MelanA/A2 specific T lymphocytes sorted with M450-AvT or M280s multimers in terms of avidity, cytoxicity and cytokine production. Avidity was evaluated by a cytotoxic assay against T2 cells loaded with increasing concentrations of the A27L Melan-A epitope. As shown in Fig. 3a, avidities of the sorted populations for the modified Melan-A epitope varied significantly from one patient to another (range of EC50 from 0.007 to 400 nM) but in a given patient, the two populations selected with M450-AvT or M280s multimers gave very similar results. Likewise, in the three patients tested, cytotoxic activity of the lymphocyte populations selected with the two types of beads against 2 HLA-A0201 + Melan-A + melanoma cell-lines, M44 and M113, and the negative control M6 HLA-A0201-melanoma cell line were remarquably similar (Fig. 3b). Thus, T lymphocytes selected with either M450-AvT or M280s multimers (both made with the mutant A27L Melan-A epitope) could recognize the natural processed Melan-A epitopes expressed on melanoma cells equally well and efficiently kill tumor cells. Finally, in two patients, we compared the capacities of the selected T cells to produce cytokines in response to melanoma cell-lines (Fig. 3c) and again found that specific lymphocytes sorted with M450-AvT produced comparable amounts of INFγ, TNFα or IL2 to that produced by M280s sorted lymphocytes.
Fig. 3.
Functional analysis of Melan-A specific T cell populations sorted with M450-AvT or M280s multimers. a Avidities of M450-AvT (filled circle) and M280s (open circle) sorted T cells for MelanA/A2 complexes was evaluated by measuring lytic activity againt the TAP-deficient cell line T2 loaded with Melan-A26–35 A27L peptide at an E:T ratio of 10:1 in a 4 h 51Cr release assay (n = 2 except for middle panel). b Lysis of melanoma cell lines by M450-AvT (black symbols) and M280s (open symbols) Melan-A sorted populations. Lysis of the HLA-A2 + Melan-A + M44 (filled triangle, open triangle), M113 (filled circle, open circle) and the HLA-A2- M6 (filled square, open square) melanoma cell lines was measured at three different E:T ratio (2:1, 10:1 and 50:1) in a 4 h 51Cr release assay. c Cytokine production of M450-AvT (black bars) and M280s (grey bars) sorted Melan-A specific populations in response to melanoma cell lines. T lymphocytes and tumor cells were mixed at a 1:2 ratio and incubated for 6 h at 37°C in the presence of Brefeldin A and stained by intracellular staining with PE-conjugated mAb against INFγ, TNFα or IL2. Percentages of cells positive for each cytokine were determined by flow cytometry
In conclusion, Melan-A/A2 specific T lymphocytes sorted with M450-AvT multimers did not functionally differ from their counterparts sorted with M280s multimers.
Application of the M450-AvT procedure to other melanoma antigens
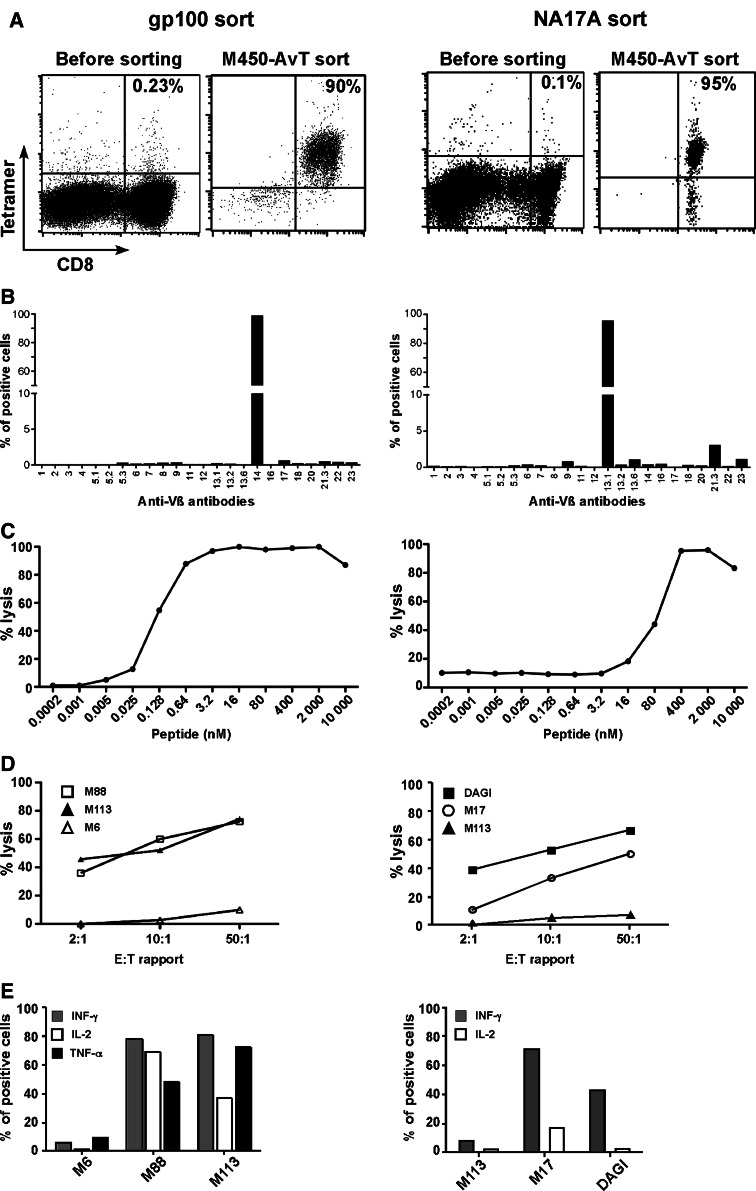
It is now well established that the T lymphocyte repertoire against the Melan-A26–35 epitope presented in HLA-A2 is well represented in the periphery of melanoma patients and it is thus relatively easy to select and expand T lymphocytes against this epitope from patients’ TIL or PBMC, as we [12, 19] and others [20, 21] have documented. It is an entirely different matter when other melanoma antigens such as gp100 or NA17-A and their related epitopes are considered because specific T cells in patients’ PBMC are often very scarce. In these latter cases, our previous experience indicated that multiple restimulations with peptide pulsed DC were necessary to expand a small fraction of specific T cells [22, 23]. We were thus prompted to test whether our procedure which relies on a single peptide stimulation before the sort would be applicable to gp100 or NA17-A epitopes. Efficiency of coating of the two monomers on M450-AvT assessed by flow cytometry was similar to that obtained with HLA-A2/Melan-A A27L (data not shown). In Fig. 4, we present two examples of sort of PBMC with M450-AvT beads coated with HLA-A2/gp100280–288 (left column) or HLA-A2/NA17-A peptide (right column). The sort of gp100-stimulated PBMC was very efficient since 90% of the population positively stained with a HLA-A2/gp100 tetramer following sort and polyclonal amplification (Fig. 4a). The small fraction of CD8−/tetramer− cells seen on the plot corresponded to remaining feeder cells that disappeared in long-term culture. Analysis of Vβ usage revealed the predominance of a single Vβ14 suggesting that the sorted population was almost monoclonal. The sorted population had a high avidity for the gp100 peptide loaded on T2 cells with an EC50 around 0.1 nM. Moreover, the selected population efficiently killed the two HLA-A2 + gp100 + melanoma cell-lines M113 and M88 but not the HLA-A2− M6 cell-line. Finally, a high fraction of the sorted lymphocytes produced TNFα, INFγ and IL2 in response to the relevant melanoma cell-lines (Fig. 4e).
Fig. 4.
Selection of GP-100 (left panel) and NA17-A (right panel) specific T cells with M450-AvT multimers. a HLA-A*0201/GP100 or NA17-A monomers coated on M450-AvT were used to sort specific T cells from peptide stimulated PBMC from a melanoma patient. After in vitro ployclonal amplification, percentages of specific T cells were assessed by double staining with PE-conjugated tetramer and FITC-conjugated anti-CD8 antibody. b Repertoire diversity of sorted populations was assessed by labeling with 24 anti-Vb antibodies. c Avidities of M450-AvT sorted T cells for GP100 or NA17-A peptide were evaluated on peptide-loaded T2 cells as in Fig. 3. d Lysis of melanoma cell lines expressing both HLA-A2 and the relevant antigen [M113 (filled triangle) and M88 (open square) for gp100 sort and M17 (open circle) and DAGI (black square) for NA17-A] along with negative controls [M6 (open triangle) for HLA-A2 and M113 (filled triangle) for NA17-A] was measured at three differents E:T ratio (2:1, 10:1 and 50:1) by a 4 h 51Cr release assay. e Cytokine production of sorted cells in response to melanoma cell-lines was evaluated as in Fig. 3 [INFγ (grey square), IL2 (open square), TNFα (black square)]
Comparable results were obtained when we sorted NA17-A specific T cells from PBMC with M450-AvT beads coated with HLA-A2/NA17-A (Fig. 4, right column). Although NA17-A specific cells were very scarce after the 14 day stimulation with peptide (0.1%), a highly enriched population (95%) could be obtained after a single sort/amplification cycle. This population was composed of a major fraction of Vβ13.1 lymphocytes but other Vβ were also present (Vβ21.3, Vβ23). The avidity of the population for NA17-A loaded onto T2 cells was rather low (EC50 around 100nM) but nevertheless these lymphocytes could kill NA17-A-expressing melanoma cell-lines (DAGI and M17) but not M113 that does not express NA17-A (Fig. 4d). A significant fraction of the population produced INFγ in response to the relevant cell-lines but only few cells produced IL2, likely due to the relatively low avidity of the population for NA17-A. The absolute numbers of specific cells recovered and the enrichment factors for the two sorts are indicated in Table 1.
These experiments confirmed that the procedure could be used to sort and expand cytotoxic T cells directed against melanoma antigens other than Melan-A.
Induction of apoptosis by multimers made with M450-AvT versus M280 beads
Previous publications have reported that crosslinking of TCR with HLA/peptide tetramers induced a significant level of activation induced cell death (AICD) [8, 24]. We therefore measured the level of apoptosis induced by M450-AvT versus M280 beads coated with HLA-A2/Melan-A26–35 A27L on Melan-A specific T cell clones. To evaluate the influence of the quantity of HLA complexes, we chose to coat M280 beads at three concentrations: M280 were either saturated with HLA complexes (M280s) (Fig. 1b) or coated with an amount (0.3 μM) that corresponded to the same quantity of complexes obtained with saturated M450-AvT (M280us1) or coated with an amount that corresponded to 2.5 times less fluorescence (M280us2) to match the density of complexes on saturated M450-AvT (Fig. 5a). We used either M280 and M450 saturated with irrelevant NA17-A/A2 monomers as negative controls in addition to a control without beads. T cell clones were incubated with an equal number of beads for 6 h at 37°C and then double stained with FITC-annexinV and propidium iodide. Typically, live cells remain negative for both markers whereas dying cells first express annexinV in the beginning of the apoptosis process and later become permeable to propidium iodide. A representative example on Fig. 5b shows that massive apoptosis of a Melan-A specific clone was induced by incubation with HLA-A2/Melan-A coated M280s (69 + 8 = 77% dying cells) as compared to the irrelevant NA17-A multimers (2.7 + 0.3 = 3%) that induced no more apoptosis than the control without beads (3.5%). In marked contrast, multimers made with M450-AvT-ELA induced little apoptosis (14 + 2 = 16% apoptotic cells), lower than the level of apoptosis induced by M280 coated with similar amounts of HLA complexes (M280us1) (56 + 13 = 69% dying cells) or with 2.5 less complexes (M280us2) (37 + 9 = 46% dying cells). We reproduced these results on a panel of Melan-A specific clones (Fig. 5c). The mean percentage of apoptosis induced by M450-AvT-ELA assessed by annexin V staining was significantly lower than that induced by M280s (16.8 ± 1.9% vs. 63.7 ± 7.0%, respectively, n = 8, P < 0.001) and also lower than that induced by M280us1 (49.9 ± 6.6%, n = 8, P < 0.01) but not significantly increased when compared to control without beads (5.3 ± 1.7%, n = 6, P > 0.05) or M280-NA17-A (2.9 ± 0.3%, n = 6 P > 0.05). M280us2 induced less apoptosis that M280us1 (26.9 ± 5.7%, n = 6, vs. 49.9 ± 6.6%, n = 8, P < 0.05) but above that induced with M450-AvT-ELA (16.8 ± 1.9%) although the difference did not reach significance.
Fig. 5.
a M280us1 were coated with a concentration of HLA-A*0201/Melan-A-27L monomers (grey histogram) adjusted to correspond to the amount of complexes obtained after saturation of M450-AvT beads (black histogram) whereas M280us2 (open histogram) were adjusted to give 2.5-fold less fluorescence intensity as assessed by staining with an FITC-anti-HLA/ABC mAb. MFI are indicated on the peaks. b A representative experiment in which a Melan-A specific CTL clone was incubated for 6 h at 37°C either alone (no bead) or with M450-AvT, M280s or M280us multimers at a cell:bead ratio of 1:1. Apoptosis was evaluated by double staining with propidium iodide and FITC-annexinV. c Summary of annexin-V stainings obtained with Melan-A-specific CTL clones incubated as in (b). ***P < 0.001, *P < 0.05 by Anova and Bonferroni post-test
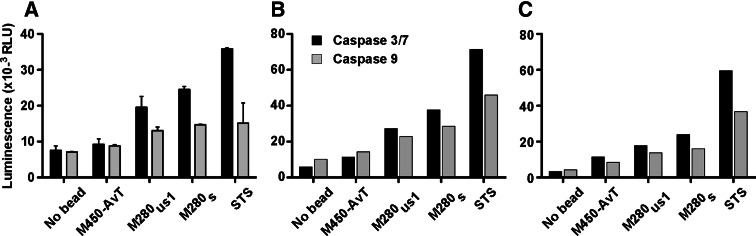
We next analyzed the activation of the caspase pathways induced by the different multimers in a Melan-A T cell clone and in two polyclonal Melan-A specific T cell-lines using staurosporin as a positive control. As shown in Fig. 6, M280s induced a major increase in caspase 9 and caspase 3/7 activities in the clone (Fig. 6a) and both specific cell-lines (Fig. 6b, c) while M280us1 induced a smaller but yet detectable increase in caspase activities. In contrast, M450-AvT induced very little caspase activation when compared to the negative control in each case (Fig. 6). These results further documented that M450-AvT multimers induced very little apoptosis as compared to M280 multimers, even when M280 were coated with a comparable quantity of HLA complexes.
Fig. 6.
Activation of caspase 3/7 and caspase 9 by M450-AvT, M280s and M280us1 multimers or staurosporine (STS) was evaluated after a 4 h incubation at 37°C at a cell:bead ratio of 1:1. After cell lysis, expression of active caspase-3/7 (black bars) and 9 (grey bars) was evaluated with the Caspase-Glo 3/7 and 9 assay. Luminescence intensity expressed as relative luminescence units (RLU) was measured with a betaplate counter. Caspase activity was tested in a Melan-A specific T cell clone in three independent experiments (a) and in two Melan-A specific T cell lines (b, c)
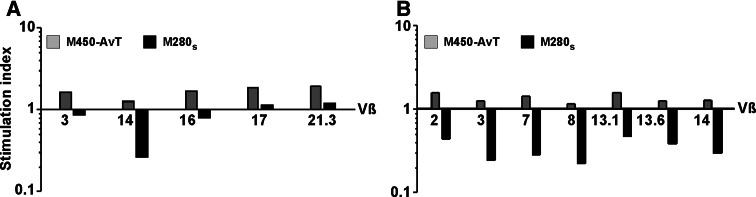
We finally tested whether the various Vβ families within the two Melan-A-specific T cell populations would respond differently to multimer stimulation in terms of proliferation/apoptosis. To this end, we compared the absolute numbers of cells within each Vβ subfamily following a 40 h incubation of the whole Melan-A specific population with M280s or M450-AvT versus “no bead”. The overall amplification factor for the two populations studied was superior to 1 following incubation with M450-AvT (1.46 for the first population in Fig. 7a and 1.60 for the second in Fig. 7b) while it was markedly inferior to 1 after incubation with M280s (0.6 and 0.36 for populations 1 and 2, respectively). The stimulation index thus reflected the balance between apoptosis and proliferation within each Vβ family. As shown in Fig. 7, M450-AvT induced the expansion of all Vβ families although the stimulation index differed from one family to the other. In contrast, following stimulation with M280s, apoptosis dominated proliferation in most Vβ subfamilies with again some variability among the different Vβ families. For example, the Vβ14 family in panel A was the most sensitive to M280-induced apoptosis and proliferated less in response to M450-AvT as compared to the Vβ17 or Vβ21.3 families. Similar observations could be made with the second Melan-A specific population with some Vβ showing particular sensitivity to apoptosis (Vβ3 and Vβ8, Fig. 7b). However, sensivity to AICD did not seem to be dictated by Vβ usage since Vβ3 specific T lymphocytes from patient 1 (Fig. 7a) were fairly resistant to apoptosis induced by M280 multimers whereas Vβ3 from the second patient were very sensitive to AICD. These results thus suggested that within the repertoire of Melan-A/A2 specific T cells in a given patient, some Vβ subfamilies ar more sensitive to multimer-induced AICD than others but we could not relate this higher sensitivity with the intensity of tetramer staining, the avidity for peptide loaded T2 cells or the reactivity towards melanoma cell-lines (data not shown).
Fig. 7.
Two different Melan-A specific T cell lines (a) and (b), were incubated for 40 h at 37°C in medium alone or with M450-AvT or M280s multimers at a cell:bead ratio of 1:1. Percentages of the main Vβ subfamilies were measured by flow cytometry and absolute numbers of each Vβ were calculated in each experimental condition. Results are expressed as stimulation indexes (SI), dividing numbers of cells expressing a given Vβ after M450-AvT or M280s stimulations by those obtained in medium alone. Grey bars indicate SI following incubation with M450-AvT and black bars incubation with M280s
Activation of cytokine production by multimers made with M450-AvT versus M280 beads
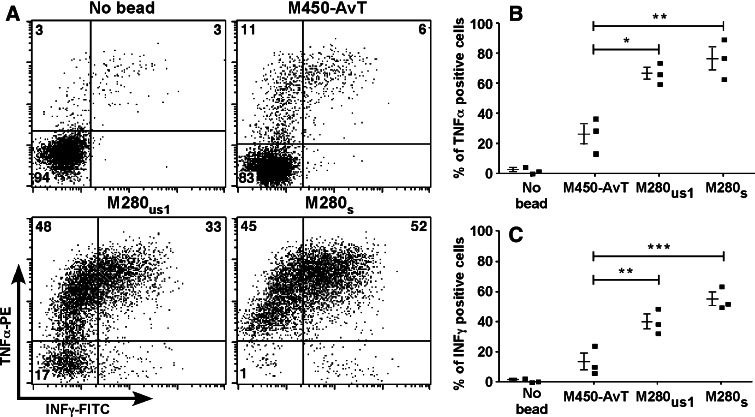
Following the observation of a low apoptosis induced by M450-AvT multimers, we wondered whether this phenomenon was restricted to apoptosis or could be extended to other activation processes triggered by TCR crosslinking such as cytokine production. We thus tested the production of TNFα and INFγ by three different Melan-A specific cell lines incubated with the three types of HLA-A2/Melan-A multimers (M280s, M280us1 and M450-AvT). A representative experiment is presented in Fig. 8a. It can be seen that M280s induced TNFα production in 97% of the cells among which 52% also produced INFγ while M450-AvT induced TNFα production in only 17% of the cells with 6% also producing INFγ.
Fig. 8.
Activation of Melan-A specific cell-lines by M450-AvT, M280s or M280us1 multimers was assessed by evaluating INFγ and TNFα production by intracellular staining following a 6 h incubation at a 1:1 ratio in the presence of brefeldin A. a A representative experiment showing double staining in the four conditions. Percentages of cells in each quadrant are indicated. b Summary of the results obtained with three distinct Melan-A specific cell lines in terms of percentages of cells producing TNFα (b) and INFγ (c). ***P < 0.001, **P < 0.01, *P < 0.05 by ANOVA and Bonferroni post-test
Again, quantity of HLA complexes on the beads could not solely explain this difference since M280us1 induced TNFα production in 81% of specific lymphocytes with 33% also producing INFγ. A summary of experiments performed on the three different cell-lines confirmed these differences: M450-AvT induced significantly less cells to produce TNFα (26.3 ± 6.8 vs. 76.7 ± 7.6, P < 0.001) or INFγ (13.3 ± 5.4 vs. 54.8 ± 4.3, P < 0.001) than M280s and also significantly less than M280us1 (26.3 ± 6.8 vs. 66.7 ± 4.1, P < 0.001 for TNFα and 13.3 ± 5.4 vs. 39.8 ± 4.8, P < 0.01 for INFγ) (Fig. 8b). When compared to the negative control without beads, M450-AvT induced a detectable increase in TNFα-producing cells and to a lesser extent of INFγ-producing cells (Fig. 8b) although the difference was not statistically significant due to the small sample size. We thus concluded that cytokine production was not totally absent following incubation with M450-AvT but yet significantly reduced as compared to M280s or M280us1.
Discussion
In the present study, we evaluated the efficiency of a new sorting procedure that aims at selecting T lymphocytes specific for a given HLA/peptide complex using only reagents that can be approved for clinical use. We thus modified our previously described procedure [11, 12] that relied on M280 streptavidin beads that are not available in clinical grade (Dynal) coated with biotinylated HLA/peptide complexes and we used instead M450-epoxy beads (clinical grade) covalently coupled to a monoclonal antibody generated against the AviTag™ peptide and then coated with non biotinylated HLA/peptide complexes. In addition to the fact that all the components of the new procedure can be obtained in clinical grade, it also allows to bypass the biotinylation of HLA complexes which is expensive, time-consuming and decreases the yield of recovery of properly loaded HLA complexes.
The major consequence of using a mAb in place of streptavidin was that we could bind a much smaller amount of HLA/peptide complexes onto the beads as assessed by staining with an anti-HLA ABC antibody (Fig. 1b). Two factors may have accounted for this decreased binding: the first factor is likely the lower affinity of the mAb AvT-6A8 for the AviTag (Kd = 6 × 10−9 M) as compared to the affinity of streptavidin for biotin (Kd = 10−13 M) [25]. The second factor, suggested by the discrepancy between the high level of antibody coupling and the small amount of bound HLA complexes, is that only a small fraction of the antibody was accessible to HLA complexes due to random coupling of the antibody to the bead by any primary amine residue. We were thus prompted to ask the critical question: Will this low amount of HLA/peptide on the beads be sufficient to bind specific T cells and will this binding be stable enough to allow efficient magnetic sorting? We provide evidence that multimers made with M450-AvT were as efficient as M280 multimers to obtain pure Melan-A specific T cell populations after a single sort/amplification cycle. Moreover, when we applied M450-AvT multimers to the sort of very rare T cell populations such as gp100 or NA17-A specific T cells (Fig. 4), we succeeded in obtaining pure specific T cell-lines after a single sort/amplification cycle whereas our previous experience using M280 multimers to sort NA17-A specific T cells showed that two sorts were usually required to achieve purity [22]. The main advantage of this great sorting efficiency is that with most melanoma antigens, a single in vitro stimulation of the patient’s whole PBMC with peptide should be enough to obtain sortable specific T cells. In terms of culture time and biological safety, a single peptide stimulation thus represents a major improvment over the procedure used by most research teams which is based on multiple restimulations with peptide loaded presenting cells (autologous irradiated PBMC or DC) [23, 26]. Moreover, we have previously shown that multiple stimulations with peptide could lead to a loss of some reactive clonotypes in culture [12]. Altogether, our data on sorting efficiencies thus suggest that M450-AvT multimers are at least as efficient as M280s multimers and possibly even better when dealing with very rare T lymphocytes in the starting population because of the minimal apoptosis induction, as discussed below.
The second concern was whether M450-AvT multimers would select only a fraction of the reactive repertoire due to their lower number of HLA/peptide complexes as compared to T cells sorted with M280 multimers. The comparison of Vβ usage among Melan-A specific T lymphocytes sorted with the two types of beads did not support this hypothesis since it did not reveal any major difference in TCR diversity between the two sorted populations. Analysis of avidities, tumor reactivity and cytokine production did not reveal any major difference either between M450-AvT and M280s sorted T cells. In addition, the sort of gp100 specific T lymphocytes produced a Vβ14 population with very high avidity for the peptide on T2 cells while the NA17-A sorted population displayed a low avidity for HLA2/NA17-A complexes (Fig. 4). Thus, sorts with M450-AvT multimers selected specific T cells with a wide range of avidities with no obvious bias in terms of avidity/reactivity.
Another major concern that has been raised about the use of HLA/peptide complexes in tetrameric or multimeric forms to label and sort specific T cells is the induction of activation induced cell death due to excessive TCR cross-linking [8, 9]. In the present report, we document that M280s multimers induced massive apoptosis of 6 Melan-A specific T cell clones after only 4 h of contact in culture (Fig. 5) that increased up to 80% after 24 h for some clones (not shown) whereas M450-AvT induced a much lower level of apoptosis that did not significantly differ from the spontaneous apoptosis observed without beads. We confirmed those marked differences in apoptosis induction by showing strong caspase 3/7 and caspase 9 activations on a Melan-A specific clone and two Melan-A specific T cell-lines by M280s multimers while M450-AvT induced very little caspase activation. The involvment of caspase activation in HLA multimer-induced cell death is still a matter of debate and conflicting reports have been published on this matter with a first report implicating Fas in the apoptotic process induced by HLA/peptide octamers [8] and a second report describing a caspase independent necrotic process [9]. Our present data support the involment of caspase 9 in the apoptotic process induced by HLA/peptide multimers i.e. the mitochondrial dependent formation of the apoptosome that will eventually lead to caspase 3/7 activation [27] but we could not determine whether the main pro-apoptotic event following TCR engagement was death receptor engagement [28] or production of reactive oxygen species by the mitochondria [29]. In fact, our attempts to detect death receptor-dependent caspase 8 activation using the Promega detection kit were unsuccessful even when we stimulated the cells with recombinant TRAIL (data not shown). Regardless of the detailed mechanisms involved in apoptosis triggering after TCR crosslinking, we observed that the significant differences between the three types of multimers in terms of AICD induction were associated with similar differences in terms of T cell activation as assessed by cytokine production. In fact, M450-AvT induced very little TNFα or INFγ in three Melan-A specific T cell-lines as compared to the production triggered both by M280s and M280us. This obervation is in agreement with the report of Cebecauer et al showing that the level of T cell apoptosis induced by HLA multimers correlated with T cell activation [9].
Our present data suggest that two main parameters concerning HLA/peptide multimers conditioned the level of induced AICD and activation i.e. the number of HLA/peptide complexes coated on the beads and/or the surface density of these complexes. When we adjusted the amount of HLA/peptide loaded on M280us1 to correspond to the amount on saturated M450-AvT beads, we observed a significant decrease in apoptosis induction as compared to that obtained with saturated M280s suggesting that the degree of apoptosis/activation was in part dependent on the total number of available HLA/peptide complexes on the beads. Considering the difference in diameter of the two types of beads (2.8 μm for M280 and 4.5 μm for M450) and thus in their surfaces (98.5 μm2 for M280 and 254.5 μm2 for M450) the same amount of HLA/peptide complexes coated on both beads will result in a density of complexes on M450 that will be roughly 2.5 times less than on M280 beads. This is the reason why we also tested the degree of apoptosis induced by M280 coated with 2.5 times less HLA/peptide complexes to match the density of M450-AvT (M280us2). We observed that these M280us2 still had a tendency to trigger more apoptosis than M450-AvT in some clones although the difference did not reach significance. Since activation will only occur if at least two crosslinked TCRs are close enough as elegantly demonstrated with dimeric HLA complexes of different lengths [24], we propose that the main reason for the little induction of apoptosis and/or activation by M450-AvT multimers is that they crosslinked TCRs that were too far apart on the T cell membrane (possibly in distinct lipid rafts) to trigger any downstream signalling. Indeed, because of steric hindrance, it is highly unlikely that both valences of a covalently bound AvT-6A8 molecule would be available for binding a HLA/ peptide monomer and thus form a stimulating dimer. In contrast, with M280 beads, even coated at very low density like M280us2, binding of two monomers on the same streptavidin is more likely to occur and form small patches of activating complexes.
Other technical solutions have been proposed to prevent activation and apoptosis by HLA multimers such as for example reversible multimers (Streptamers) that could be detached from specific T cells after fluorescence cell-sorting by competition with biotin [30, 31] but this technology will be very difficult to validate in a clinical setting.
In contrast, the new sort/amplification procedure that we present in this report will allow the fast and efficient isolation of specific T lymphocytes with little induction of TCR signalling and can be easily adapted to a clinical settings. We hope that this technology will help to boost the evaluation of passive immunotherapy by specific T cell infusion in cancer.
Acknowledgments
This work was supported by a grant from the «Ligue Nationale contre le Cancer» (labellisation 2003–2007) and by a grant from INCa “Thérapie adoptive cellulaire du cancer”.
Abbreviations
- ACT
Adoptive cell therapy
- AICD
Activation induced cell death
- CMV
Cytomegalovirus
- EBV
Epstein–Barr virus
- GMP
Good manufacturing practice
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic cell
- HLA
Human leucotyte antigen
- PBMC
Peripheral blood mononuclear cells
- TCR
T cell receptor
- TIL
Tumor inflitrating lymphocyte
References
- 1.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 2.Gustafsson A, Levitsky V, Zou JZ, Frisan T, Dalianis T, Ljungman P, Ringden O, Winiarski J, Ernberg I, Masucci MG. Epstein–Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 3.Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, Lin YF, Quiros-Tejeira RE, Reinke P, Schubert S, Gottschalk S, Finegold MJ, Brenner MK, Rooney CM, Heslop HE. Treatment of solid organ transplant recipients with autologous Epstein–Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 5.Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S, Cassidanius A, Lemarre P, Billaudel S, Labarriere N, Jotereau F. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B, Jotereau F. Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother. 2002;51:532–538. doi: 10.1007/s00262-002-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillaume P, Legler DF, Boucheron N, Doucey MA, Cerottini JC, Luescher IF. Soluble major histocompatibility complex-peptide octamers with impaired CD8 binding selectively induce Fas-dependent apoptosis. J Biol Chem. 2003;278:4500–4509. doi: 10.1074/jbc.M208863200. [DOI] [PubMed] [Google Scholar]
- 9.Cebecauer M, Guillaume P, Hozak P, Mark S, Everett H, Schneider P, Luescher IF. Soluble MHC-peptide complexes induce rapid death of CD8 + CTL. J Immunol. 2005;174:6809–6819. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 10.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, Olavarria E, Goldman J, Chakraverty R, Mahendra P, Craddock C, Moss PA. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodinier M, Peyrat MA, Tournay C, Davodeau F, Romagne F, Bonneville M, Lang F. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat Med. 2000;6:707–710. doi: 10.1038/76292. [DOI] [PubMed] [Google Scholar]
- 12.Labarriere N, Gervois N, Bonnin A, Bouquie R, Jotereau F, Lang F. PBMC are as good a source of tumor-reactive T lymphocytes as TIL after selection by Melan-A/A2 multimer immunomagnetic sorting. Cancer Immunol Immunother. 2008;57:185–195. doi: 10.1007/s00262-007-0361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gervois N, Heuze F, Diez E, Jotereau F. Selective expansion of a specific anti-tumor CD8 + cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- 14.Jotereau F, Pandolfino MC, Boudart D, Diez E, Dreno B, Douillard JY, Muller JY. LeMevel B (1991) High-fold expansion of human cytotoxic T-lymphocytes specific for autologous melanoma cells for use in immunotherapy. J Immunother. 1991;10:405–411. doi: 10.1097/00002371-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 16.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 17.Guilloux Y, Lucas S, Brichard VG, Van Pel A, Viret C, De Plaen E, Brasseur F, Lethe B, Jotereau F, Boon T. A peptide recognized by human cytolytic T lymphocytes on HLA-A2 melanomas is encoded by an intron sequence of the N-acetylglucosaminyltransferase V gene. J Exp Med. 1996;183:1173–1183. doi: 10.1084/jem.183.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vignard V, Lemercier B, Lim A, Pandolfino MC, Guilloux Y, Khammari A, Rabu C, Echasserieau K, Lang F, Gougeon ML, Dreno B, Jotereau F, Labarriere N. Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J Immunol. 2005;175:4797–4805. doi: 10.4049/jimmunol.175.7.4797. [DOI] [PubMed] [Google Scholar]
- 19.Gervois N, Labarriere N, Le Guiner S, Pandolfino MC, Fonteneau JF, Guilloux Y, Diez E, Dreno B, Jotereau F. High avidity melanoma-reactive cytotoxic T lymphocytes are efficiently induced from peripheral blood lymphocytes on stimulation by peptide-pulsed melanoma cells. Clin Cancer Res. 2000;6:1459–1467. [PubMed] [Google Scholar]
- 20.Cole DJ, Wilson MC, Rivoltini L, Custer M, Nishimura MI. T-cell receptor repertoire in matched MART-1 peptide-stimulated peripheral blood lymphocytes and tumor-infiltrating lymphocytes. Cancer Res. 1997;57:5320–5327. [PubMed] [Google Scholar]
- 21.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8 + T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 22.Labarriere N, Bretaudeau L, Gervois N, Bodinier M, Bougras G, Diez E, Lang F, Gregoire M, Jotereau F. Apoptotic body-loaded dendritic cells efficiently cross-prime cytotoxic T lymphocytes specific for NA17-A antigen but not for Melan-A/MART-1 antigen. Int J Cancer. 2002;101:280–286. doi: 10.1002/ijc.10605. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Linette GP, Longerich S, Haluska FG. Antimelanoma activity of CTL generated from peripheral blood mononuclear cells after stimulation with autologous dendritic cells pulsed with melanoma gp100 peptide G209–2 M is correlated to TCR avidity. J Immunol. 2002;169:531–539. doi: 10.4049/jimmunol.169.1.531. [DOI] [PubMed] [Google Scholar]
- 24.Cebecauer M, Guillaume P, Mark S, Michielin O, Boucheron N, Bezard M, Meyer BH, Segura JM, Vogel H, Luescher IF. CD8 + cytotoxic T lymphocyte activation by soluble major histocompatibility complex-peptide dimers. J Biol Chem. 2005;280:23820–23828. doi: 10.1074/jbc.M500654200. [DOI] [PubMed] [Google Scholar]
- 25.Freitag S, Le Trong I, Klumb L, Stayton PS, Stenkamp RE. Structural studies of the streptavidin binding loop. Protein Sci. 1997;6:1157–1166. doi: 10.1002/pro.5560060604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutoit V, Rubio-Godoy V, Dietrich PY, Quiqueres AL, Schnuriger V, Rimoldi D, Lienard D, Speiser D, Guillaume P, Batard P, Cerottini JC, Romero P, Valmori D. Heterogeneous T-cell response to MAGE-A10(254–262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 2001;61:5850–5856. [PubMed] [Google Scholar]
- 27.Creagh EM, Conroy H, Martin SJ. Caspase-activation pathways in apoptosis and immunity. Immunol Rev. 2003;193:10–21. doi: 10.1034/j.1600-065X.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- 28.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 29.Gulow K, Kaminski M, Darvas K, Suss D, Li-Weber M, Krammer PH. HIV-1 trans-activator of transcription substitutes for oxidative signaling in activation-induced T cell death. J Immunol. 2005;174:5249–5260. doi: 10.4049/jimmunol.174.9.5249. [DOI] [PubMed] [Google Scholar]
- 30.Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B, Bernhard H, Wagner H, Busch DH. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002;8:631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 31.Neudorfer J, Schmidt B, Huster KM, Anderl F, Schiemann M, Holzapfel G, Schmidt T, Germeroth L, Wagner H, Peschel C, Busch DH, Bernhard H. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Meth. 2007;320:119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]