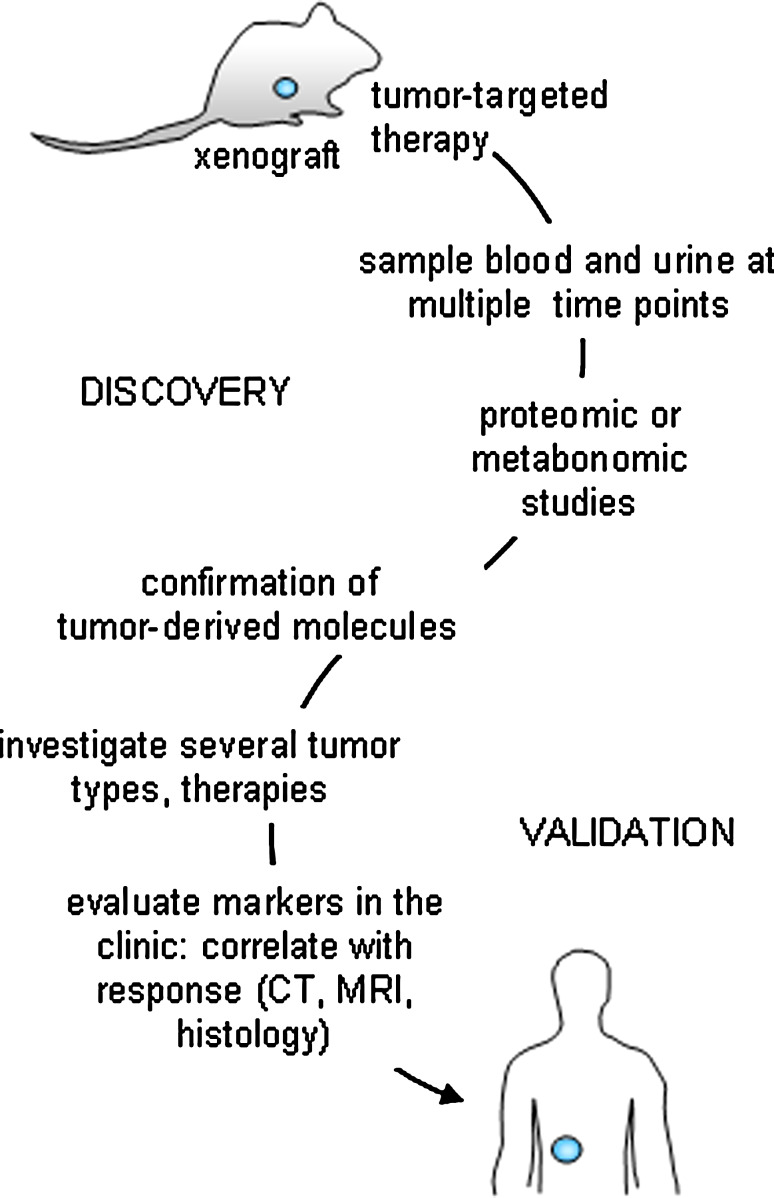
Fig. 3.
Scheme for validating response markers as measures of true clinical outcome. Initially, the study of human tumor specimens engrafted into immunocompromised mice will be most useful for the identification of candidate markers of tumor response when treated with a targeted therapy (e.g. Apo2L/TRAIL, Herceptin®). Once identified and confirmed with proteomic and/or metabonomic studies, other more non-specific therapies can be investigated (e.g. chemotherapy, radiation). Inquiries into whether the biomarkers are tumor or treatment specific will be elucidated. Once established, clinical studies will identified whether the same molecules are detectable in patients during therapy. Importantly, cohorts of patients will need to be followed for assessment of tumor response with traditional techniques such as tumor imaging and histology and these evaluations will need to be correlated with the newly identified therapeutic efficacy markers to assess their measurement of true clinical response