Abstract
Tumor-derived immunosuppressive factors contribute to the evasion of malignant cells from the immune response, partially by hampering dendritic cell (DC) differentiation. Here, we analyze whether soluble mediators released by the most frequent histological types of non-small cell lung carcinoma, squamous cell carcinoma (SCC), and adenocarcinoma (AD) cells, affect the development and functionality of DC. Monocytes from healthy donors were differentiated in vitro into DC with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4, in the absence or presence of soluble factors (SF) from SCC or AD cell lines. Monocytes were differentiated in parallel into macrophages (MΦ s) with macrophage colony-stimulating factor (M-CSF). SF-treated DC were phenotypically and functionally more similar to MΦ s than to untreated DC [control DC (Ctrl-DC)]. Both tumors increased myelomonocytic markers (CD14, CD16, CD32, and CD163) and impaired CD1a expression on DC. SF-treated DC increased their endocytic capacity, and released higher levels of IL-6, IL-10, and lower levels of IL-12, compared to Ctrl-DC. SF-treated DC were poor stimulators in mixed lymphocyte reactions, and naïve CD4+ T lymphocytes stimulated by SF-treated DC secreted lower levels of interferon (IFN)-γ and higher amounts of IL-10 than controls. In contrast to AD, the effects caused by SCC were mostly abolished by IL-6 neutralization during monocyte differentiation. However, tumor-derived prostanoid blockade recovered the IFN-γ levels secreted by lymphocytes stimulated with SF-treated DC, whereas prostanoid/IL-6 or prostanoid/IL-10 blockade decreased IL-10 production only by SCC-DC-stimulated lymphocytes. Thus, we provide evidence that lung SCC and AD cause comparable deficiencies on DC in vitro, skewing monocyte differentiation from DC to MΦ -like cells, but most of these changes occurred via different mediators.
Keywords: Lung carcinoma, Dendritic cell differentiation, Interleukin-6
Introduction
Worldwide, lung cancer is the leading cause of death by malignant diseases [1]. Despite the advances in diagnosis and treatment in the past two decades, the prognosis of lung cancer is still poor [2]. Non-small cell lung carcinoma (NSCLC) accounts for 70–80% of all lung cancer cases. They represent a heterogeneous group of tumors, consisting mainly of squamous cell carcinoma (SCC), adenocarcinoma (AD), and large cell carcinoma [3]. The first two histopathological types of NSCLC show the highest incidence.
The main obstacles for further improving survival of patients with lung cancer are the lack of effective methods for early detection and strategies for treating patients with advanced tumors. The high incidence and mortality rates of NSCLC have led to design adjuvant therapies to reduce tumor progression [4]. Immunotherapy protocols based on the use of monocyte-derived dendritic cells (DC), which have succeeded with some tumors, show limited responses in NSCLC patients [5]. DC are the most powerful antigen presenting cells (APCs) of the immune system, and are recognized by their unique ability to activate T-cell primary responses. The poor results of DC-treated NSCLC patients may be explained in part by the low immunogenicity of tumor-associated antigens. However, lung cancer patients show defects in DC function [6], which could result in tumor evasion from the immune response. Furthermore, in vitro studies have demonstrated inhibitory effects caused by non-well-defined lung tumor-derived suppressor factors acting on DC [7–9]. Though it is unknown how these failures in DC function take place in vivo, and the molecules or mechanisms involved remain elusive.
Monocytes recruited into inflamed tissues are precursors of DC and macrophages (MΦ) in vivo [10]. Recently, Rotta et al. [11] demonstrated that activation of T lymphocytes in vivo against particulate antigens is mainly dependent of monocyte-derived DC, confirming the biological relevance of these cells. The balance of DC/MΦ differentiation from monocytes in situ is affected by the tissue microenvironment. Tumor growth factors such as interleukin (IL)-10 or IL-6 [12], which are secreted at high levels during the establishment and development of some tumors, favor monocyte differentiation toward the MΦ pathway and prevent the generation of DC [13]. Furthermore, improved immune responses are frequently associated with the presence of mature DC in the tissues [14], whereas an abundance of MΦ correlated with diminished responses and, in the case of tumors, with progressive disease [15, 16].
Tumor and tumor-infiltrating cells in the lung produce a variety of pro-inflammatory factors and anti-inflammatory factors, such as transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, IL-6, IL-8, or prostanoids [17, 18], and some of them are involved in the pathogenesis and progression of lung carcinomas. In NSCLC patients, high IL-6 serum levels are associated with tumor progression, lower response to chemotherapy, and short survival rate [19, 20]. In addition, augmented IL-6 receptor (IL-6R) intracellular signaling activity is related with enhanced survival of NSCLC cells [12], and over-expression of IL-6 in transfected lung-cancer cell lines increases tumor replication in vivo [21]. However, some reports have demonstrated that primary lung SCC preferentially express IL-6 compared with primary lung AD [17].
Here, we hypothesized that soluble factors (SF) produced by lung carcinoma cells might affect the differentiation or functionality of DC. Thus, the aim of this study was to compare the effects of conditioned medium from AD to SCC cell lines on human monocyte-derived DC phenotype and function.
Materials and methods
Media and reagents
Tumor cell lines were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, (Hyclone Laboratories, Logan, UT, USA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol (Gibco BRL, Grand Island, NY, USA), referred as complete medium. For monocyte culture, 5% heat-inactivated pooled AB human serum from healthy volunteers was added. The following reagents were used: human recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF, 1000 U/ml), interferon (IFN)-γ (1000 U/ml), rat neutralizing monoclonal antibody (mAb) to IL-10 (JES3-19F1, 5 μg/ml) and its Ig isotype control (BD PharMingen, San Diego, CA, USA); human IL-2 (20 U/ml, PeproTech, Rocky Hill, NJ, USA); human IL-4 (15 ng/ml), and macrophage colony-stimulating factor (M-CSF, 30 ng/ml) (Calbiochem, La Jolla, CA, USA); mouse neutralizing mAb to IL-6 (6708, 5 μg/ml), to TGF-β1 (9016, 5 μg/ml) and their respective Ig isotype controls (R&D Systems, Minneapolis, MN, USA); lipopolysaccharide (LPS) from Escherichia coli 0111:B4 (LPS, 0.5 μg/106 cells/ml, Sigma-Aldrich, St Louis, MO, USA); cyclooxygenase (COX)-1/-2 inhibitor indomethacine (10 μM, Cayman Chemical, Ann Arbor, MI, USA); carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, Eugene, OR, USA); tetanus toxoid (TT, 1 μg/ml, kindly supplied by the National Institute of Hygiene, Mexico).
Lung carcinoma cell lines
The following lung carcinoma cell lines were used: SK-MES-1 and Calu-1 as representative of SCC, and A-549, A-427, and SK-LU-1 as representative of AD, all from the ATCC (Rockville, MD, USA). The AD cell line 3B1A, obtained from a patient and established in our laboratory, was also included in this study. Peripheral blood mononuclear cells (PBMC) from healthy donors were used as negative control cells. Conditioned media (CM) were obtained by culture of carcinoma cell lines (1×106 cells) or PBMC (5×106 cells) in 10 ml of complete medium for 24 h, as it has been reported [7]. Occasionally, CM was prepared in the presence of indomethacine. CM was recovered after centrifugation to remove the cells and frozen at −80°C.
Cell separation and differentiation of peripheral blood monocytes
Peripheral blood mononuclear cells were isolated from buffy coats of healthy volunteers using Lymphoprep (Axis-Shield, Oslo, Norway) density gradient centrifugation. CD14+ monocytes were separated by positive selection with magnetic cell sorting (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany). Allogeneic naïve CD4+ CD45RO− T lymphocytes and autologous memory CD4+ CD45RA− T cells from TT-vaccinated individuals were isolated by negative selection using MACS CD4+ T-cell isolation kit (Miltenyi Biotec), followed by incubation with MACS anti-CD45RO mAb or anti-CD45RA mAb, respectively.
Isolated monocytes were cultured at 106 cells/ml supplemented with GM-CSF and IL-4 to obtain DC or with M-CSF to generate MΦ . The cultures were fed with fresh medium and cytokines every 2 days. When used, TT was added to DC at fifth day of culture. After 6 days, non-adherent DC or MΦ were harvested. To assess the effect of lung-carcinoma supernatants on DC differentiation, 50% of the culture medium [7] was replaced with CM from SSC or AD cell lines, and added every 2 days along with fresh medium and cytokines. As a negative control, CM from PBMC was supplied to the cells. In some assays, neutralizing mAb to IL-6, IL-10, or TGF-β1 were added to the cultures, and replaced together with fresh media and cytokines every 2 days. Morphological changes were documented by inverted phase microscopy (Olympus IMT-2, Japan).
Immunofluorescence assays
The following mAb were used: anti-HLA-A,B,C (G46-2.6), anti-HLA-DR (TU36), anti-CD1a (HJ149), anti-CD11b [ICRF44(44)], anti-CD14 (M5E2), anti-CD16 (3G8), anti-CD32 [FLI8. 26(2003)], anti-CD40 (5C3), anti-CD80 (BB1), anti-CD83 (HB15a), anti-CD86 (IT2.2), anti-CD163 (Ber-MAC3), and anti-CD206 (19.3), all from BD PharMingen except CD163 (Dako, Carpinteria, CA, USA) and CD83 (Immunotech, Marseille, France). After 6 days of culture, DC were stained with the above mAb followed by FITC-labeled goat F(ab′)2 anti-mouse Igs polyclonal antibody (Dako). Endocytic activity of DC and MΦ was assessed by uptake of soluble FITC-coupled dextran (Mr=40,000, Molecular Probes), as was described previously [22]. Samples were analyzed on a FACSCalibur using the CellQuest software (Becton Dickinson, Mountain View, CA, USA).
Cytokine detection
Total RNA was isolated from (2.5–5)×105 DC, MΦ, or from 1×106 tumor cell lines, using Trizol reagent (Gibco BRL). cDNA was synthesized by reverse transcription and PCR was performed as described previously [22]. β-Actin was amplified as an internal control, and the ratios of cytokine/β-actin band densities were determined in order to evaluate the relative expression of each cytokine mRNA. Oligonucleotide primers and the expected sizes of PCR products were published elsewhere [22, 23]. Production of IL-6, IL-10, and IL-12 p70 in the supernatants of DC or MΦ was quantified by ELISA kits from BD PharMingen. Cells were incubated for 24 h with LPS and IFN-γ at the concentrations mentioned before. Controls were established with cells cultured for 24 h without additional stimulus. TGF-β1, TNF-α, IL-6, and IL-10 production by lung carcinoma cell lines was measured with ELISA kits (BD PharMingen) from 24-h supernatants of 1×106 cells/10 ml.
T-cell activation
For proliferation assays, allogeneic PBMC from healthy donors (3×105) were seeded into 96-well round-bottom microplates and cultured with graded numbers of stimulator cells (DC or MΦ) in complete medium. Proliferation of T cells was measured on day 5 after an 18-h pulse with [3 H] thymidine (0.5 μCi/well; Amersham Biosciences Corp., Piscataway, NJ, USA). For T-cell memory lymphocyte proliferation assays, lymphocytes were labeled with CFSE as described [24] and co-cultured with TT-pulsed DC at 8:1 ratio for 5 days. Then, lymphocytes were labeled with phycoeritrin (PE)-conjugated CD25 (M-A251, BD PharMingen) and analyzed by flow cytometry.
For cytokine production assays, (2–4)×105 naïve or memory T cells were co-cultured with allogeneic or TT-pulsed DC, respectively, at 10:1 ratio in complete medium. Controls were set without addition of DC. After 5 days of priming, T cells were expanded with IL-2 for additional 5 days. Lymphocytes were then washed extensively and restimulated for 24 h with immobilized anti-CD3 mAb (5 μg/ml, UCHT1, BD PharMingen) for quantitation of cytokine secretion. Measurement of IFN-γ, IL-4, and IL-10 in the supernatants was performed by ELISA kits from BD PharMingen.
Statistical analyses
Data were expressed as mean ± SD of independent experiments. The statistical significance of the data was determined by the Student’s two-tailed paired t-test, assuming equal variances. ANOVA test was used for pairwise multiple comparisons.
Results
Cytokine mRNA relative expression and secretion by lung carcinoma cell lines
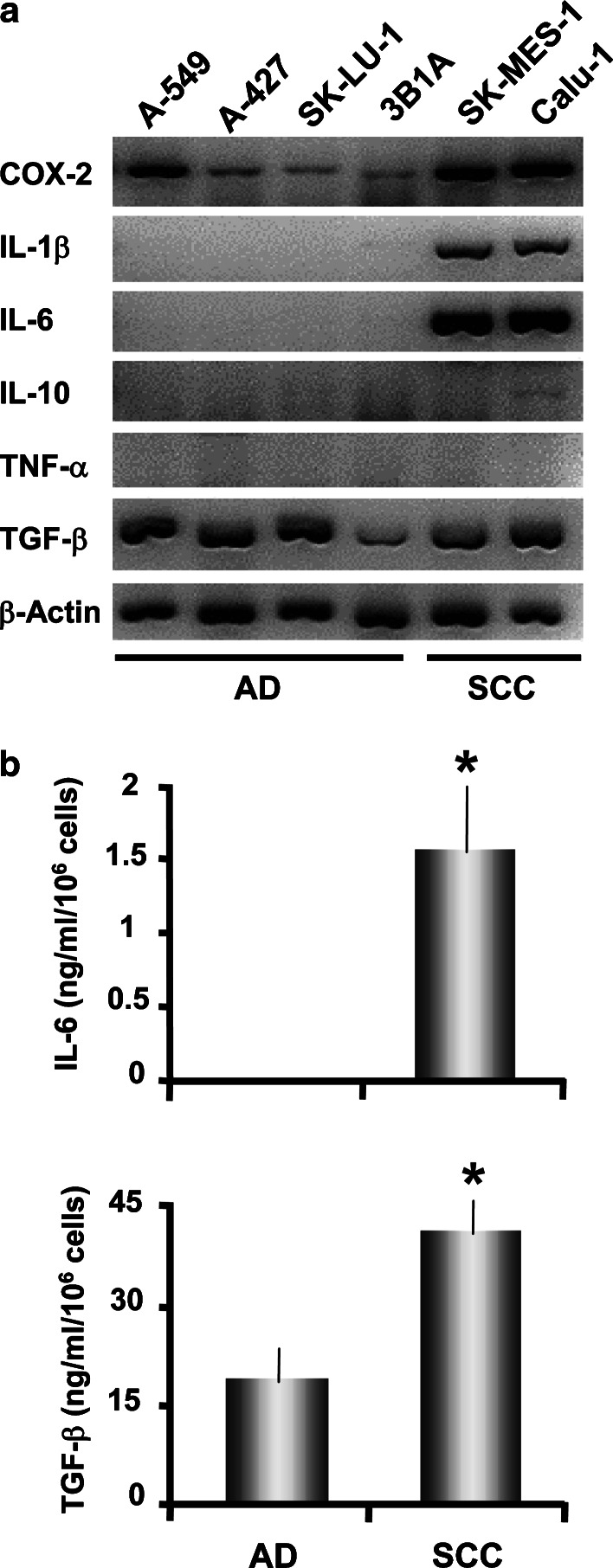
Relative expression of COX-2, IL-1β, IL-10, IL-6, TNF-α, and TGF-β transcripts was studied in AD and SCC cell lines. All lung carcinoma cell lines expressed TGF-β transcripts and protein as was previously reported (Fig. 1a, b; Ref. [25]), and they constitutively expressed COX-2 mRNA, but SCC cell lines had higher levels of transcripts. Furthermore, SCC cells secreted higher levels of TGF-β compared with AD cells (Fig. 1b, p<0.05). Conversely, we did not detecte IL-10 or TNF-α mRNA (Fig. 1a) and protein (data not shown) in these tumor cell lines. In addition, IL-1β transcripts and IL-6 transcripts and protein were detected in SCC but not in AD cell lines (Fig. 1a, b, p<0.05), in accordance with previous reports [17].
Fig. 1.
a, b Cytokine and COX-2 detection on different SCC and AD cell lines. a AD cell lines (A-549, A-427, SK-LU-1, and 3B1A) and SCC cell lines (SK-MES-1 and Calu-1) were cultured at 106 cells/10 ml for 24 h. Then, total RNA was extracted and RT-PCR for the indicated molecules and β-actin was performed. Agarose gel electrophoresis of RT-PCR fragments is shown from a representative experiment. b Analysis of IL-6 and TGF-β1 production by A-427 (AD) and Calu-1 (SCC) cell lines. Cells were harvested after 24 h of culture, and cytokines in the supernatants were evaluated by ELISA. Results are the mean ± SD of three independent experiments. Statistical analysis (AD versus SCC): * p<0.05
CM from AD or SCC cell lines induce alterations in DC morphology and phenotype
To characterize the effects caused by SF derived from lung AD or SCC cells on DC differentiation, human monocytes were differentiated into DC in the presence of CM derived from several AD (AD-DC) or SSC (SCC-DC) cell lines. Untreated DC or DC differentiated with PBMC-CM were used as controls. In the subsequent experiments, since no significant difference on DC function or phenotype was detected between the two control conditions (not shown), we will refer to them indistinctly as control DC (Ctrl-DC). Given the influence of the cytokines secreted by the lung carcinoma cell lines analyzed here on DC differentiation from monocytes [13], we also included controls of monocyte-derived MΦ for comparison.
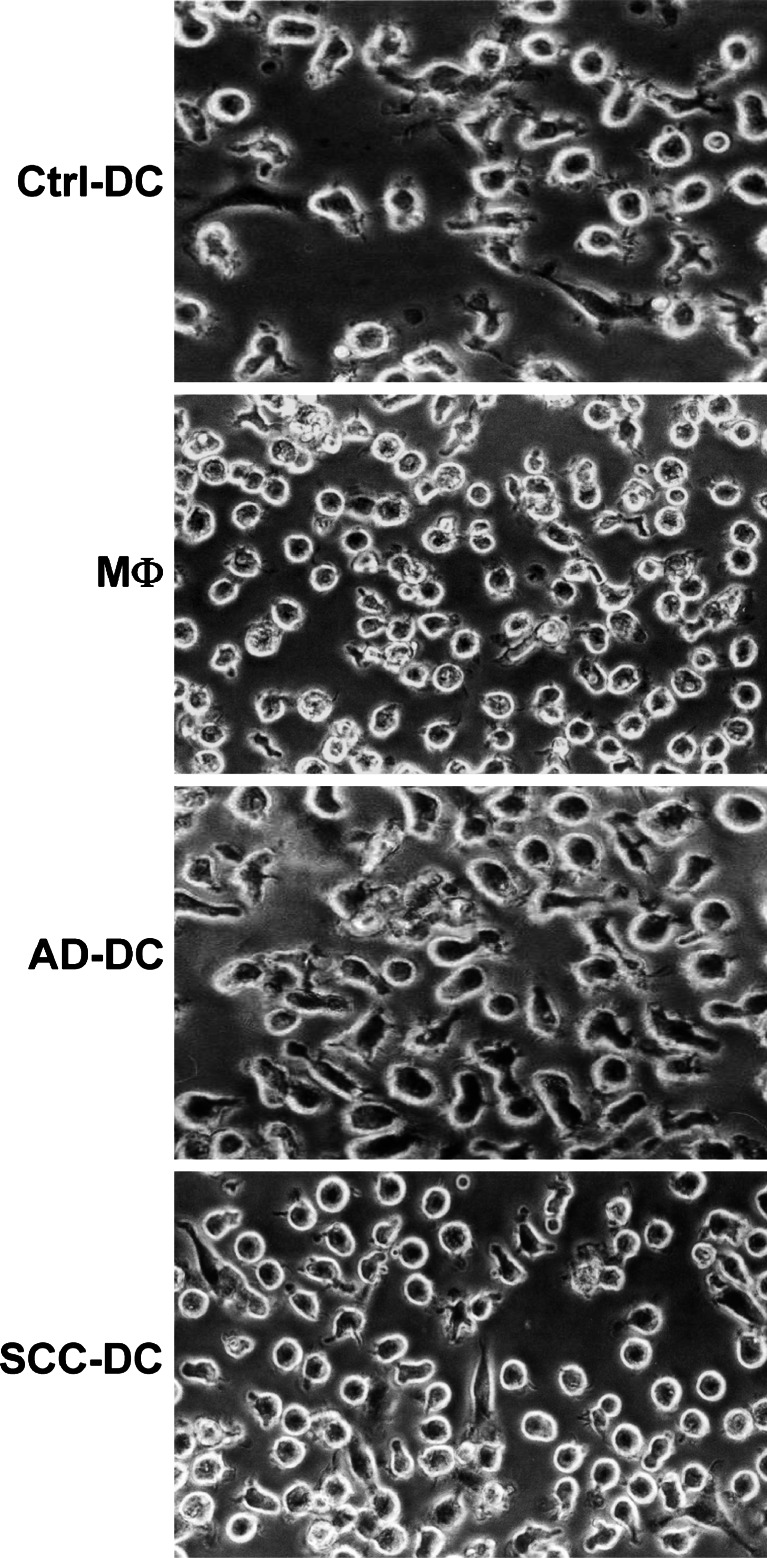
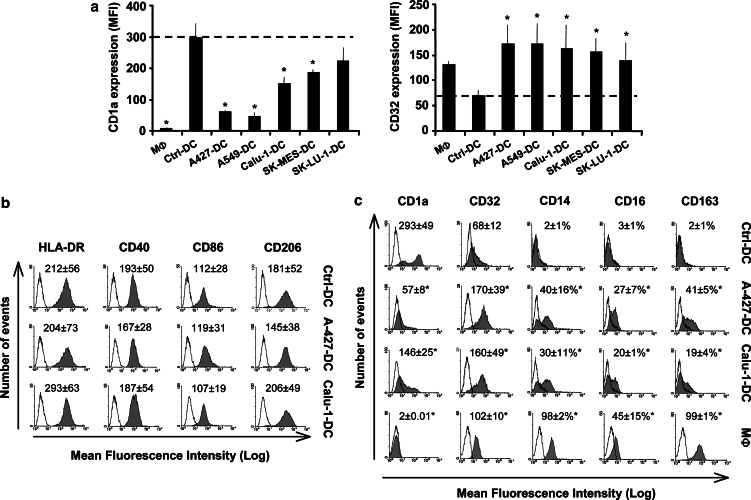
Morphological, phenotypic, and functional changes induced by AD-CM or SCC-CM were analyzed at the end of the DC differentiation process (day 6 of culture). Tumor cell-treated DC presented morphological modifications compared to Ctrl-DC (Fig. 2). A number of AD-DC and SCC-DC acquired a rounded appearance similar to MΦ differentiated in vitro with M-CSF, which was more evident in SCC-DC. With regard to phenotypic changes in AD-DC and SCC-DC, we detected a diminution of CD1a and an increase of CD32 expression to different degrees in DC treated with CM from all the tumor cell lines tested compared to Ctrl-DC (Fig. 3a), analogous to the expression found in MΦ . According to these results, and in order to simplify the analysis of the biological effects of AD-CM and SCC-CM on DC differentiation, the following results will show alterations induced by Calu-1 (as representative of SCC cell lines) and A-427 (as representative of AD cell lines). No changes in the surface expression of HLA-DR, CD40, CD86, CD206 (mannose receptor) (Fig. 3b), HLA class I, CD11b, CD80, or CD83 (not shown) were observed in tumor cell-treated DC compared to controls. A significant increase (p<0.05) of myelomonocytic markers such as CD14, CD16, CD32, and CD163 (a member of the scavenger receptor family) was detected in tumor cell-treated DC with respect to Ctrl-DC (Fig. 3c). In addition, CD1a expression was greatly diminished in AD-DC (by fivefold) and to a lesser extent in SCC-DC (by twofold) (Fig. 3c, p<0.05). Overall, the phenotype of tumor cell-treated DC showed more similarities to monocytes/MΦ than to Ctrl-DC (Fig. 3c).
Fig. 2.
Conditioned media (CM) derived from lung AD or SCC cell lines induce changes on DC morphology. Monocytes were differentiated into DC or MΦ, and DC were treated with PBMC supernatants as a control (Ctrl-DC), or with A-427 (AD-DC) and Calu-1 (SCC-DC) supernatants. Morphology under the inverted phase microscope of the resulting cell populations is shown
Fig. 3.
a–c Phenotypic changes induced by tumor-derived CM on DC. a CD1a and CD32 expression on MΦ and DC cultured in the absence (Ctrl-DC) or presence of supernatants from AD (A-427-DC, A-549-DC, SK-LU-1-DC) and SCC cell lines (Calu-1-DC and SK-MES-1-DC). Results are presented as the mean ± SD of five independent donors. b HLA-DR, CD40, CD86, and CD206 expression (gray histograms) on Ctrl-DC, A-427-DC, and Calu-1-DC, analyzed by flow cytometry. Mean of fluorescence intensity (MFI) for each marker is indicated on the top of the histograms. Empty profiles represent isotype-matched irrelevant antibodies used as negative controls. c Phenotype analysis as in b including a representative result of MΦ . MFI for CD1a and CD32 and the percentage of positive cells for CD14, CD16, and CD163 are indicated on the top of the histograms. MFI and percentage of positive cells in b and c are the mean ± SD of five donors. Results shown are from a representative experiment. Statistical analysis (MΦ or tumor cell-treated DC versus Ctrl-DC): * p<0.05
Increase of endocytic capacity on tumor cell-treated DC
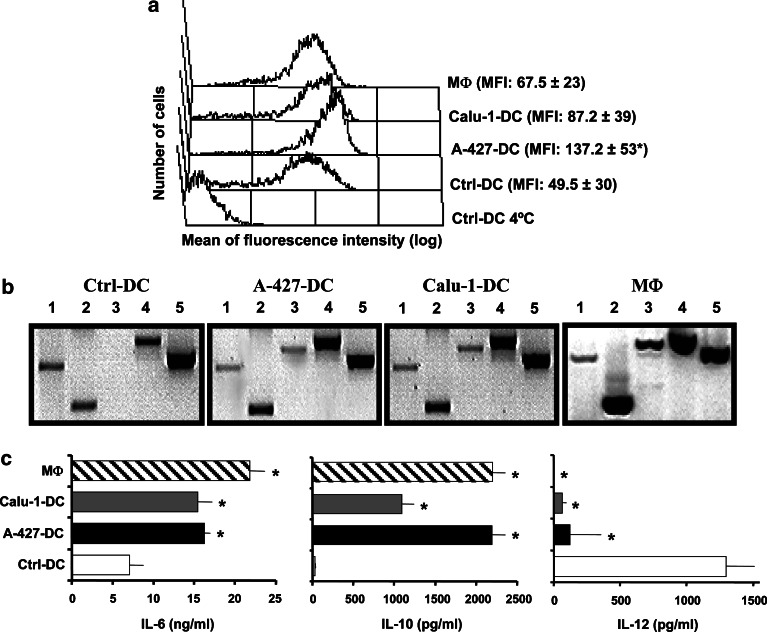
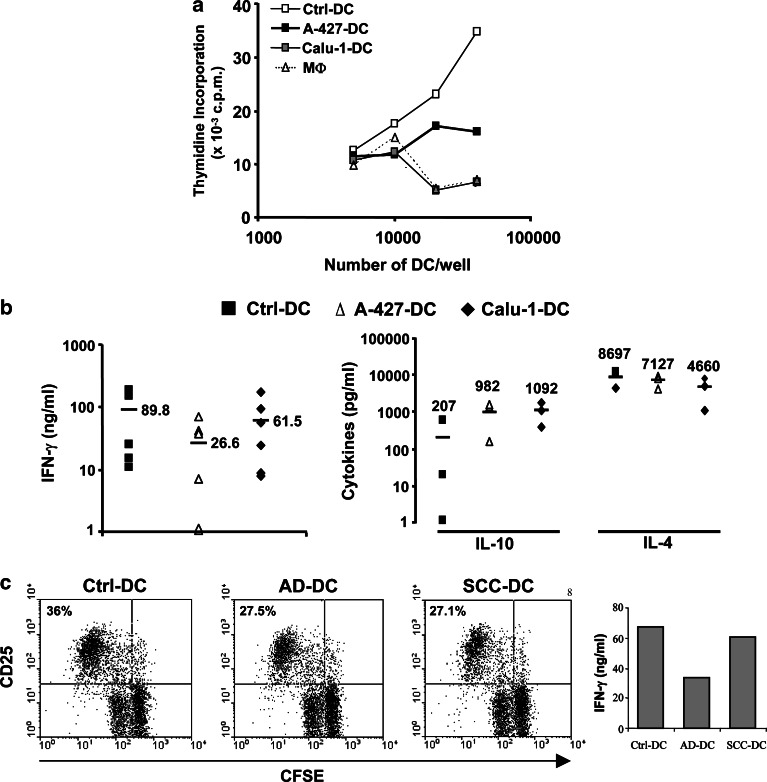
Dendritic cell differentiated in the presence of CM derived from tumor cell lines increased their capacity to uptake FITC-dextran (Fig. 4a). AD-DC accumulated roughly threefold more dextran (MFI: 137.2±53) than Ctrl-DC (MFI: 49.5±30, p<0.05). SCC-DC internalized approximately twofold more antigen (MFI: 87.2±39) than Ctrl-DC (not significant). MΦ showed an endocytic ability similar or slightly superior (MFI: 67.5±23) than Ctrl-DC (Fig. 4a).
Fig. 4.
a–c Functional characterization of tumor cell-treated DC. a Endocytic activity of DC differentiated in the presence of CM from tumor cell lines. Ctrl-DC, A-427-DC, Calu-1-DC, and MΦ were incubated with FITC-dextran at 37°C or at 4°C as a control of internalization, and then analyzed by flow cytometry. The experiment shown is representative of four similar performed ones. Values to the right are the mean ± SD of FITC incorporation of the independent experiments. b Cytokine mRNA detection on the above cell populations. RT-PCR for the following molecules was performed: TNF-α (lane 1), IL-10 (lane 2), IL-6 (lane 3), TGF-β1 (lane 4), and β-actin (lane 5). Shown is an agarose gel electrophoresis of RT-PCR fragments in a representative experiment out of five. c Cytokine secretion by Ctrl-DC (white bars), A-427-DC (black bars), Calu-1-DC (gray bars), and MΦ (hatched bars). Cells were stimulated with LPS plus IFN-γ, and the supernatants were harvested after 24 h. Production of IL-6, IL-10, and IL-12 was evaluated by ELISA. Data shown are the mean ± SD of four independent experiments. Statistical analysis (MΦ or tumor cell-treated DC versus Ctrl-DC): * p<0.05
Tumor cell-treated DC modify their cytokine mRNA expression and protein secretion profile
The relative expression of transcripts for IL-6, IL-10, IL12 p40, TNF-α, and TGF-β in DC cultured in the presence or absence of CM derived from carcinoma cell lines was evaluated (Fig. 4b and Table 1). Under steady-state conditions, no detectable levels of IL-12 p40 mRNA were found (data not shown). A decreased relative expression of TNF-α transcripts was observed in tumor cell-treated DC compared to Ctrl-DC (p<0.05 with AD-DC); in contrast, an increased expression of IL-10 (p<0.05 with AD-DC) and TGF-β transcripts was detected. De novo expression of IL-6 mRNA was found in SCC-DC and AD-DC (p<0.05). The monocyte-derived MΦ population showed a cytokine mRNA expression profile similar to tumor cell-treated DC.
Table 1.
Relative expression of cytokine mRNA in DC and MΦ
| TNF-α | TGF-β1 | IL-6 | IL-10 | |
|---|---|---|---|---|
| Ctrl-DC | 0.47±0.02 | 0.35±0.29 | 0.00±0.01 | 0.27±0.30 |
| A-427-DC | 0.08±0.07*, a | 0.80±0.15* | 0.20±0.13* | 0.45±0.04 |
| Calu-1-DC | 0.25±0.70 | 0.85±0.9 | 0.50±0.19* | 0.6±0.14 |
| MΦ | 0.1±0.07* | 1.5±0.18* | 0.60±0.3* | 1.6±0.2* |
RT-PCR was performed as indicated in Fig. 4b. Bands from agarose gel electrophoresis were quantified by densitometry, and the data were individually normalized to the values of β-actin. Results are presented as mRNA amount in arbitrary units (mean ± SD of four independent experiments)
a Statistical analysis (A-427-DC, Calu-1-DC and MΦ versus Ctrl-DC): * p<0.05
Dendritic cell from different treatments and MΦ were stimulated with LPS and IFN-γ in order to induce cytokine secretion. A statistically significant (p<0.05) reduction of IL-12 production was detected in tumor cell-treated DC compared to Ctrl-DC (Fig. 4c). In contrast, IL-6 and IL-10 secretion was significantly increased (p<0.05) in both SCC-DC and AD-DC (Fig. 4c). MΦ produced similar levels of IL-12, IL-6, and IL-10 than tumor cell-treated DC (Fig. 4c).
T lymphocyte stimulation by tumor cell-treated DC
Despite tumor cell-treated DC had equivalent expression of antigen presentation and costimulatory molecules than Ctrl-DC, their differential production of cytokines such as IL-10 or IL-12 prompted us to study the capacity of these DC to induce allogeneic T-cell proliferation. As shown in Fig. 5a, the immunostimulatory capacity of DC previously treated with SCC-CM or AD-CM was lower with respect to DC treated with Ctrl-CM, and analogous to what was found by using MΦ as APCs. Further, we analyzed the influence of tumor cell-treated DC on the extent of allogeneic naïve CD4+ T-cell polarization (Fig. 5b). Primed CD4+ T cells stimulated by AD-DC secreted lower amounts of IFN-γ compared to controls (n=6, p=0.01). The average of IFN-γ secretion by SCC-DC-stimulated lymphocytes tended to be lower than controls, but differences were not significant. Moreover, T cells stimulated by tumor cell-treated DC showed a great increase of IL-10 secretion, which was significant with SCC-DC (n=3, p<0.05). No major changes on IL-4 levels were observed (n=3).
Fig. 5.
a–c T-cell function is modified after stimulation with tumor cell-treated-DC. a Lymphoproliferative capacity of Ctrl-DC, A-427-DC, Calu-1-DC, and MΦ in a mixed lymphocyte reaction. Graded doses of the above cells were incubated with 3×105 allogeneic PBMC for 5 days, followed by an 18-h pulse of [3 H] thymidine. One experiment out of four is shown. Results are presented as [3 H] incorporation (c.p.m) and are averages of triplicates. b Production of IFN-γ, IL-10, and IL-4 after co-cultures of allogeneic naïve CD4+ CD45RO− T lymphocytes with Ctrl-DC (filled squares) or with DC treated with CM from A-427 (open triangles) or Calu-1 (filled diamonds) cell lines, as described in Materials and methods. Cytokines in culture supernatants were analyzed by ELISA. Each symbol represents the result of a single donor, and average values are indicated with horizontal bars and numbers. c Stimulatory ability of DC in recalls responses. TT-pulsed DC were cultured for 5 days with CFSE-labeled autologous memory CD4+ T cells, stained with CD25-PE and analyzed by flow cytometry. Numbers in the dot plots represent percentages of CD25+ proliferating cells. Right IFN-γ production by T cells in the same experiment
Memory T-cell responses against TT were also affected when the antigen was presented by tumor cell-treated DC (Fig. 5c). T cells stimulated with AD-DC or SCC-DC showed slightly lower rates of proliferation, but their expression of CD25 was similar, compared with Ctrl-DC. Nevertheless, TT-specific memory T cells primed with AD-DC still secrete lower levels of IFN-γ than Ctrl-DC (Fig. 5c).
Blockade of IL-6 during monocyte differentiation with tumor-CM restores DC phenotype
A variety of tumor-derived immunosuppressive factors have been involved in alterations of DC development from human monocytes: vascular endothelial growth factor [26], IL-6 [13, 27], IL-10 [28, 29], TGF-β1 [30] or prostaglandin (PG) E2 [31]. According to our results, A-427 cells secrete TGF-β1, and their production of PGE2 has been reported [32]. On the other hand, Calu-1 cells secrete IL-6 and TGF-β1. Finally, tumor cell-treated DC had the ability to secrete IL-10 (see Fig. 4c). Therefore, we consider these factors as candidates to cause the changes detected on AD-DC and SCC-DC. Their role was evaluated by neutralizing IL-6, IL-10, and TGF-β1 during DC differentiation with mAb. COX-1/2-derived prostanoid activity was assessed by treatment of tumor cells with indomethacine for 24 h before CM was harvested.
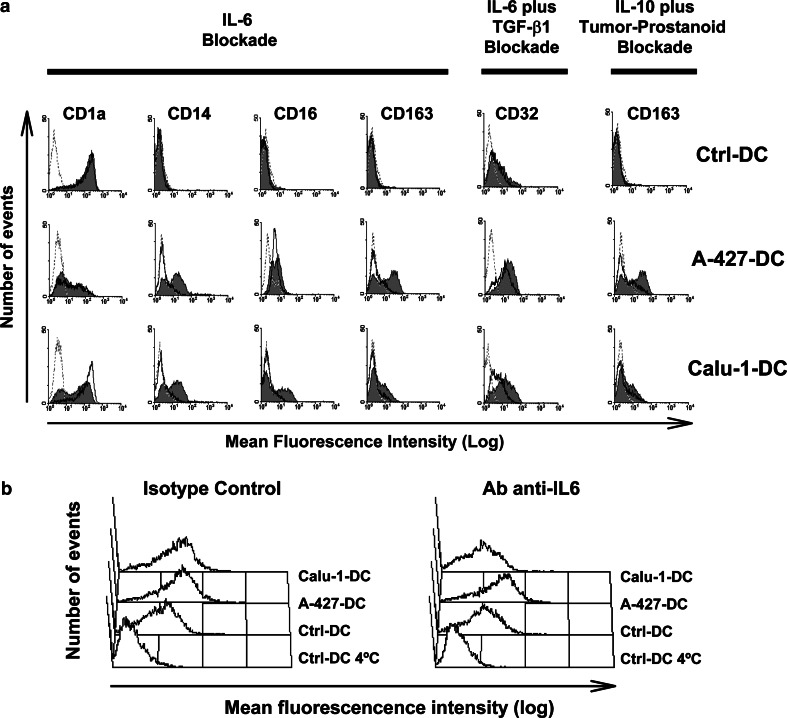
The use of neutralizing mAb to IL-6, mAb-IL-10 or mAb-TGF-β1, isotype-matched control mAb, or CM derived from indomethacine-treated PBMC, did not modify Ctrl-DC phenotype (Fig. 6a, and data not shown). IL-6 blockade during monocyte differentiation with SCC-CM completely reverted cell phenotype, and we found expression of CD1a, CD14, CD16, CD32, and CD163 to the levels of Ctrl-DC (Fig. 6a). CD32 diminution was partially dependent on TGF-β1 (Fig. 6a), since IL-6 alone did not cause this effect (not shown). In AD-DC, IL-6 neutralization reverted CD14 and CD163 expression to the levels shown by Ctrl-DC (Fig. 6a). The effect of IL-6 on CD163 was reproduced when IL-10 and prostanoids were neutralized (Fig. 6a). A slight decrease on CD32 expression was also observed on AD-DC attributable to a synergistic effect of both IL-6 and TGF-β1 neutralization (Fig. 6a). The blockade of IL-10, TGF-β or prostanoids alone did not reestablish the phenotypic alterations on both AD-DC and SCC-DC (not shown).
Fig. 6.
a, b Effect of IL-6, IL-10, TGF-β1, and tumor-derived prostanoids on DC phenotype and endocytic activity. Monocytes were cultured for 6 days with GM-CSF and IL-4, in the presence of PBMC-CM (Ctrl-DC), AD-CM (A-427-DC) or SCC-CM (Calu-1-DC). At the beginning of cultures, anti-IL-6, anti-IL-10, or anti-TGF-β1 neutralizing mAb, and their corresponding isotype-matched control mAb, were added. Tumor-derived prostanoid blockade was achieved by incubating tumor cell lines with indomethacine for 24 h. Control CM for these supernatants was obtained from PBMC after the same treatment. Cells received the treatments alone or in combination. a Surface marker expression of cells treated with isotype-matched control mAb and/or indomethacine-PBMC-CM (solid gray histograms) or with neutralizing mAb and/or indomethacine-tumor-CM (empty black histograms). Treatments and the markers analyzed are indicated on the top. Dotted histograms represent the fluorescence of an irrelevant isotype-matched control mAb for each marker. Shown is a representative experiment out of three. b Day-6 monocyte-derived cells cultured as above were incubated with FITC-dextran for 30 min at 37°C or at 4°C (as a control). After washing, fluorescent cells were evaluated by flow cytometry. Treatment of the cells is indicated on the top. Histograms are representative of one of two independent experiments
IL-6 neutralization decreased the endocytic capacity and the ability to secrete high levels of IL-6 and IL-10 on SCC-DC
The effects of IL-6, IL-10, TGF-β1 and prostanoids observed on DC phenotype indicated that they were present during monocyte differentiation with both AD-CM and SCC-CM. We then investigated their effects during monocyte differentiation on DC endocytic activity and cytokine secretion.
IL-6 neutralization decreased the antigen uptake capacity of SCC-DC to the levels of Ctrl-DC (p<0.05), whereas AD-DC were not affected (Fig. 6b). IL-10, TGF-β or tumor-derived prostanoid blockade had no effect on the endocytic ability of tumor cell-treated DC (data not shown).
IL-6 and prostanoids were responsible for the generation of DC able to secrete high levels of IL-6 and IL-10 when cells were treated with SCC-CM (p<0.05), and these factors had no synergistic effects (Table 2). Additionally, IL-10 was also responsible for the high amounts of IL-10 secreted by SCC-DC (p<0.05, Table 2), and no synergistic effect with IL-6 or prostanoids was observed (not shown). In contrast, blockade of IL-6, IL-10, TGF-β1, prostanoids, or their combinations (Table 2, and data not shown) had no effect on the high levels of IL-6 secreted by AD-DC. Among these factors, only IL-10 altered monocyte differentiation with AD-CM, by generating cells capable to produce elevated amounts of IL-10 (p<0.05). Finally, none of the factors tested restored the ability of tumor cell-treated DC to secrete high levels of IL-12 (Table 2).
Table 2.
Regulation of cytokine secretion by Ctrl-DC, A-427-DC (AD-DC), and Calu-1-DC (SCC-DC)
| Isotype Control Ab | Ab anti-IL6 | Ab anti-IL10 | Ab anti-TGF-β1 | Indomethacine | Ab anti-IL-6 plus indomethacine | |
|---|---|---|---|---|---|---|
| IL-6 | ||||||
| Ctrl-DC | 6.4±1.6 | 5.1±0.7 | 7.9±1.8 | 5.1±1.1 | 6.7±1.0 | 5.5±0.8 |
| AD-DC | 16.8±0.7*,a | 16.0±1.6 | 18.4±1.4 | 17.6±1.5 | 17.6±1.9 | 17.3±0.6 |
| SCC-DC | 15.8±1.7* | 6.4±0.5**,b | 16.8±2.6 | 16.7±2.6 | 8.0±0.5** | 7.5±0.3** |
| IL-10 | ||||||
| Ctrl-DC | 0.07±0.02 | 0.06±0.03 | 0.01±0.01 | 0.05±0.01 | 0.07±0.02 | 0.12±0.03 |
| AD-DC | 2.16±0.16* | 1.6±0.1 | 0.19±0.07** | 2.0±0.23 | 1.9±0.2 | 1.51±0.08 |
| SCC-DC | 1.4±0.15* | 0.4±0.06** | 0.36±0.18** | 1.8±0.33 | 0.4±0.1** | 0.37±0.08** |
| IL-12 | ||||||
| Ctrl-DC | 1.3±0.1 | 1.5±0.3 | 1.5±0.5 | 1.1±0.2 | 1.9±0.7 | 2.1±0.6 |
| AD-DC | 0.1±0.03* | 0.3±0.3 | 0.6±0.4 | 0.04±0.06 | 0.3±0.03 | 0.25±0.04 |
| SCC-DC | 0.09±0.04* | 0.3±0.3 | 0.1±0.03 | 0.07±0.08 | 0.2±0.2 | 0.23±0.08 |
DC were obtained as in Fig. 6. After 6 days of culture, cells were stimulated with LPS plus IFN-γ for 24 h, and then supernatants were harvested. Production of IL-6, IL-10, and IL-12 was evaluated by ELISA, and results are presented in ng/ml. Data shown are the mean ± SD of four independent experiments Statistical analysis: a* p<0.05 compared to corresponding Ctrl-DC b** p<0.05 compared to corresponding carcinoma-treated DC in the presence of isotype control mAb
Presence of IL-6, IL-10, and prostanoids during DC differentiation with tumor-CM interfere the cytokine secretion profile of DC-stimulated T lymphocytes
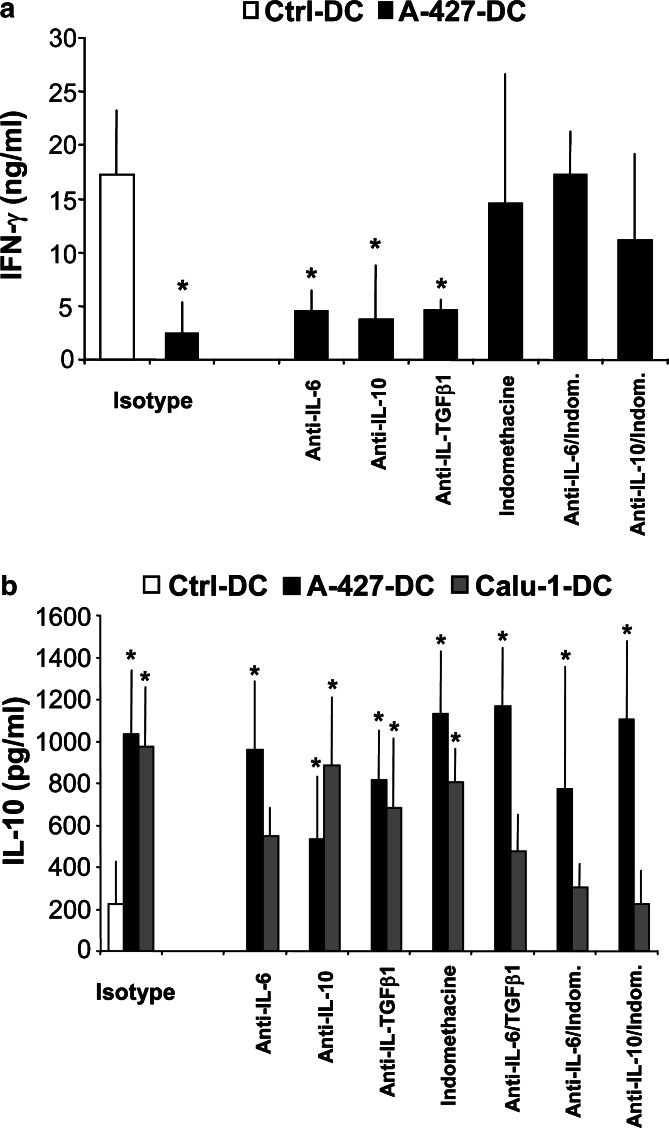
We subsequently analyze the influence of IL-6, IL-10, TGF-β, and tumor-derived prostanoids on DC capacity to induce T-helper cell polarization and lymphocyte proliferation. We found that blockade of each factor during monocyte differentiation did not modify the low capacity of tumor cell-treated DC to elicit allogeneic lymphocyte proliferation (data not shown). However, blockade of tumor-derived prostanoid production fully restored the capacity of AD-DC to induce high production of IFN-γ by allogeneic naïve CD4+ T lymphocytes (Fig. 7a). No changes were observed after IL-6, IL-10, or TGF-β1 blockade (Fig. 7a). Therefore, AD-derived prostanoids appear to be solely responsible for generating DC that induce low levels of IFN-γ on lymphocytes.
Fig. 7.
a, b Effect of IL-6, IL-10, TGF-β1, and tumor-derived prostanoids on DC ability to promote cytokine secretion on allogeneic naïve CD4+ T lymphocytes. DC were obtained as in Fig. 6, and co-cultures with T lymphocytes were established as described in Materials and methods. Cytokines in culture supernatants were determined by ELISA. a Analysis of IFN-γ secretion on culture supernatants of lymphocytes stimulated with Ctrl-DC (white bars) or A-427-DC (black bars). b Analysis of IL-10 on culture supernatants of lymphocytes stimulated with Ctrl-DC (white bars), A-427-DC (black bars) or Calu-1-DC (gray bars). DC treatments are indicated on the x-axis in a and b, and results are the mean ± SD of three independent experiments. Statistical analysis (tumor cell-treated DC versus Ctrl-DC): * p<0.05
High production of IL-10 by naïve CD4+ T lymphocytes was dependent on tumor-derived prostanoids together with IL-6 or IL-10 activity only when monocytes were differentiated in the presence of SCC-CM, but not in the presence of AD-CM (Fig. 7b). Tumor-derived prostanoids, TGF-β1 or IL-10 alone did not influence the ability of tumor cell-treated DC to induce high levels of IL-10 on T lymphocytes, while blockade of IL-6 partially diminished this effect (Fig. 7b). None of the factors tested during AD-DC differentiation had a significant influence on the high levels of IL-10 secreted by AD-DC-stimulated T lymphocytes.
Discussion
Non-small cell lung carcinoma is represented by two main histological types, AD and SCC, which have a range of similarities and differences at the genetic level, clinical manifestations, response to treatments, and survival rate of the patients [3, 4, 33]. In cancer-bearing patients, a number of tumor-derived factors have been associated with immunosuppression, partially through modifications of DC function or development [7–9]. In the present study, we compared for the first time the potential of SF released by lung AD and SCC cells to alter the differentiation of human DC.
We confirmed that both AD and SCC cells secrete TGF-β1 [25] and constitutively express COX-2 mRNA. However, SCC cell lines secrete higher amounts of TGF-β1 and express greater levels of COX-2 transcripts, which probably leads to a higher production of PGE2, a factor that critically affect DC differentiation [34]. We also observed that only the SCC cell lines tested here express IL-1β transcripts and produce IL-6, in keeping with recent reports that lung SCC secrete higher levels of IL-6 than AD cells [17, 21]. Both cytokines might be involved in the elevated levels of COX-2 mRNA expressed by SCC cells [35, 36]. Additionally, we demonstrated that neither AD nor SCC cells secrete detectable levels of IL-10, which is consistent with previous reports of human NSCLC cell lines [37]. Hence, our data support the notion that AD and SCC cells secrete common (i.e. TGF-β1) and different mediators (i.e. IL-6) with immunosuppressive capacities [30, 38].
It is noteworthy that, regardless of the difference on the type or amount of the factors released, AD and SCC cell lines cause similar phenotypic and functional alterations on cells generated by culture of circulating monocytes with GM-CSF and IL-4, and we suggest that they skew DC differentiation toward cells with MΦ characteristics. A number of differentiating DC cultured with tumor-CM showed round morphology, expressed typical monocyte/MΦ markers (CD14, CD163), inhibited the full induction of CD1a (a classical marker of monocyte-derived DC not expressed by MΦ), increased their endocytic ability, and altered their pattern of cytokine secretion analogous to what is found on monocyte-derived MΦ . Furthermore, tumor cell-treated DC (as well as MΦ) were poor stimulators of T-lymphocyte proliferation compared with DC cultured in the absence of tumor-CM, and changed the pattern of cytokine secreted by naïve T CD4+ lymphocytes after priming. Overall, these lymphocytes produced low amounts of IFN-γ and increased levels of IL-10. Memory T-cell responses were also altered by tumor cell-treated DC, being the most relevant feature the low levels of IFN-γ secretion induced by AD-DC. Taken together, the present results suggest that lung tumor-derived factors promote the commitment of monocyte precursors into the MΦ lineage. We favor the hypothesis of switching monocyte differentiation from DC to MΦ over a prevention of monocyte differentiation since they were cultured with GM-CSF, which allows monocyte differentiation, the resulting cells had larger size than monocytes (comparable to MΦ, Fig. 2), and they expressed lower levels of CD14 and higher levels of HLA-DR and CD86 than monocyte precursors (not shown). However, we cannot rule out the presence of some cells with monocyte characteristics.
In our system there is a complex interplay of factors secreted by carcinoma cell lines and those derived from GM-CSF/IL-4-treated monocytes. Differentiating DC have the potential to secrete IL-6, M-CSF [28, 39], and PGE2 [40] under steady-state conditions. IL-4 plays a pivotal role promoting monocyte differentiation into DC and blocking the alternative MΦ lineage pathway. Conversely, GM-CSF favors MΦ development via M-CSF, IL-6, and M-CSF receptor (CD115) induction. M-CSF is a key MΦ differentiation factor, but its presence in the culture does not skew monocyte differentiation from DC to MΦ [27, 28]. Other cytokines such as IL-6 or IL-10 increase CD115 expression on differentiating DC, which renders monocytes fully responsive to M-CSF, allows autocrine M-CSF consumption, and switches monocyte differentiation toward the MΦ lineage [13, 28]. In addition, tumor-derived factors such as prostanoids [31], VEGF [26], bombesin-like peptides [9], or mucins [41], can modify the characteristics of DC generated from GM-CSF/IL-4 cultured monocytes, and the magnitude of this alteration is often dependent on the concentrations of the mediators involved [34, 38].
According to our results, AD-DC produced IL-10 and IL-6 during the culture, and SCC-DC secreted at least IL-10. These cytokines were detected in supernatants of AD-DC and SCC-DC at low levels (data not shown), but most important, their neutralization prevented some of the changes induced by tumor-CM. Tumor-derived PGE2 is a likely candidate for IL-6 and IL-10 induction on DC, as well as IL-6 in the case of SCC cells [42, 43]. These and the above results suggest that at least TGF-β1, IL-6, IL-10, and prostanoids were present during monocyte differentiation with AD-CM and SCC-CM, probably at different levels according to the type of tumor. Interestingly, some of the factors found in our experimental conditions have been reported to mediate alterations on DC development or function: IL-6 and IL-10 favor the differentiation of monocytes into MΦ [13, 29], IL-10, and TGF-β generate tolerogenic or regulatory DC [44], and PGE2 down-regulates the ability of monocyte-derived DC to activate polarized Th1 responses [34].
Tumor-derived TGF-β1 was barely implicated in any of the changes observed on AD-treated DC or SCC-treated DC. In contrast, IL-10 secreted by tumor cell-treated DC stimulated its own secretion in an autocrine/paracrine manner and it was implicated in the increment of IL-10 secretion by T-lymphocytes primed with SCC-DC. The latter effect was dependent on the combined action of prostanoids [31, 34] and IL-10 or IL-6 during SCC-DC differentiation. In that sense, we suggest that SCC-derived IL-6 activates IL-10 secretion by SCC-DC [45], and the combined effect of IL-10 together with prostanoids ultimately mediates the abnormal production of IL-10 by lymphocytes. Therefore, it is possible that the high levels of prostanoids and IL-6 produced by SCC cells are the main contributors to generate DC, which prime T lymphocytes to secrete great amounts of IL-10. In contrast, the levels of prostanoids produced by AD cells might not be sufficient to alter the ability of DC to induce IL-10 on T lymphocytes, and other factors not analyzed here should produce this effect. In any case, because tumor-infiltrating regulatory T lymphocytes have been described in NSCLC [46], it is tempting to speculate that both AD-DC and SCC-DC can generate “regulatory” T cells with a high capacity to secrete IL-10 and a low rate of proliferation (see Fig. 5a).
The role of tumor-derived IL-6 in immune suppression has been studied mainly in non-solid tumors such as multiple myeloma and chronic lymphocytic leukemia [47, 48], where IL-6 hampered the development and maturation of DC. However, few studies have reported a direct effect of IL-6 secreted by solid tumors on DC differentiation or function [13, 38, 49], and its role has not been addressed in the context of NSCLC. Under in vitro conditions, we showed that lung SCC, but no AD, cell lines express and secrete IL-6, and that blockade of IL-6 activity during SCC-DC differentiation abolished most of the MΦ -like characteristics of these cells: expression of myelomonocytic markers, low induction of CD1a, high endocytic activity, and increased production of IL-6 and IL-10 after LPS/IFN-γ stimulation. These results are consistent with a role of IL-6 increasing the levels of CD115 on differentiating DC [13, 27, 49], thus it is plausible that IL-6 acts indirectly by rendering monocytes responsive to endogenously produced M-CSF. Interestingly, blockade of IL-6 activity on AD-DC also impaired the expression of some myeloid markers (CD14 and CD163), suggesting that their expression could be modulated with low levels of IL-6, whereas other markers might need a more potent signaling via IL-6R. In fact, most of the MΦ -related characteristics of AD-DC were not abolished by blocking IL-6, IL-10, TGF-β, or prostanoid activity. As was mentioned before, other factors released by tumor cells could modify DC phenotype and function. Some of these mediators (i.e. MUC1 and MUC5AC mucins) are over-expressed in AD cells [50], therefore they are potential candidates to explain the development of AD-DC.
A common characteristic of several tumor-derived immunosuppressive factors is their role on the development of DC with low production of IL-12 and high production of IL-10 [31, 34, 41, 51]. Here, we observed that tumor cell-treated DC secretes limited amounts of IL-12. Low production of IL-12 is a characteristic of MΦ derived from monocytes cultured with M-CSF [52], which reinforce the hypothesis that this cytokine could play an important role in our system. In spite of PGE2 [34, 40] or IL-10 [51] can selectively block IL-12 synthesis; our data demonstrate that none of these factors are implicated in this process. In that sense, Sombroek et al. [31] demonstrated that IL-12 secretion couldn’t be fully restored on DC treated with CM from colon carcinomas by blocking tumor-derived prostanoid activity, hence other components of the CM might act synergistically with prostanoids to reduce IL-12 production by DC. Regarding the high levels of IL-10 secreted by tumor cell-treated DC, they are dependent indistinctly on IL-6 or prostanoids in SCC-DC, whereas a mechanism that relies on the autocrine/paracrine consumption of IL-10 is predominant in AD-DC. We hypothesized that overproduction of IL-6 by SCC cells could activate autocrine PGE2 production [36], and the effect of tumor-derived IL-6 on IL-10 production by SCC-DC might be mediated indirectly by tumor-derived PGE2 [31, 53]. Conversely, AD cells may synthesize reduced levels of PGE2 based on their COX-2 mRNA expression, which could be associated with their lack of IL-6 production. In that scenario, the action of autocrine IL-10 might be predominant, although at present it is uncertain what tumor-derived mediators are responsible for IL-10 induction on AD-DC.
The finding that T lymphocytes stimulated with tumor cell-treated DC secrete low levels of IFN-γ is in keeping with the low IL-12 production by these DC [31, 34, 40]. Nevertheless, the absence of IL-12 did not completely abolish IFN-γ production [54]. Here we demonstrated that tumor-derived prostanoid activity during DC differentiation negatively influences IFN-γ production by T cells. This effect was reverted by blocking COX activity in AD cells (see Fig. 7a). A similar tendency was observed in SCC-DC (data not shown), although these cells induce only slightly reduced levels of IFN-γ compared with Ctrl-DC (see Fig. 5b). Since prostanoids do not appear to influence IL-12 levels in our system (Table 2), their effect on IFN-γ secretion could be mediated by nonidentified factors differentially secreted by SCC-DC and AD-DC.
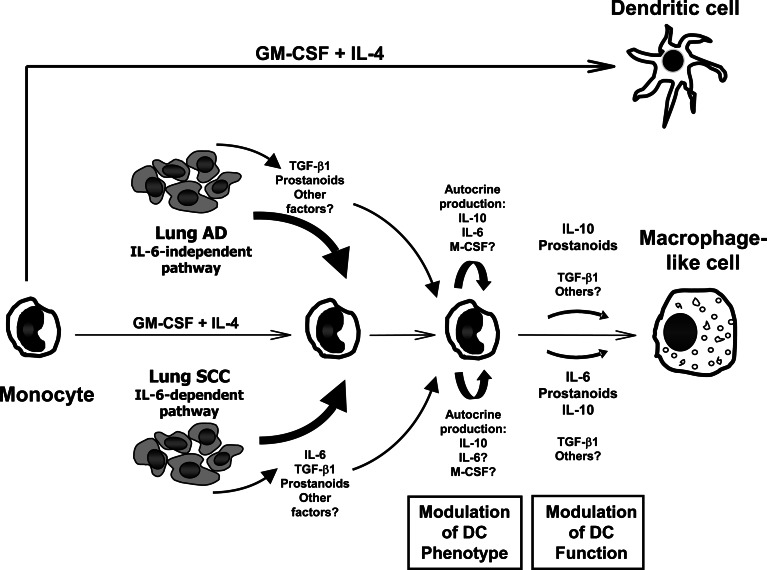
In summary, our results suggest that lung SCC and AD cells switch monocyte differentiation from DC to MΦ -like cells trough IL-6-dependent and IL-6-independent mechanisms, respectively (Fig. 8). The function of this type of MΦ -like cells might be correlated with that of tumor associated MΦ, which are involved in tumor growth and invasion [55]. Thus, based on our findings, IL-6 could play an important role in the pathogenesis of SCC partly through the suppression of an effective immune response.
Fig. 8.
Model for in vitro differentiation of monocyte-derived DC in the presence of SF released by lung AD and SCC cell lines. In standard conditions, human monocytes can generate DC by culture with GM-CSF and IL-4. However, in the presence of SF secreted by AD and SCC cells, monocyte-derived cells have characteristics similar to MΦ . Tumor cell lines produce SF such as TGF-β1 (both) and IL-6 (SCC only), among others. They do not secrete detectable levels of TNF-α or IL-10. Furthermore, it is likely that both tumor types produce PGE2, since they constitutively express COX-2. Tumor cell-treated DC increased their levels of IL-6, IL-10, and TGF-β1 transcripts. Overall, TGF-β1 exerts little effect on the system. In contrast, most of the changes observed on SCC-DC could be attributable to IL-6 secreted by SCC cell lines. IL-6 increases CD115 (M-CSF receptor) expression in differentiating DC [13], rendering cells responsive to endogenously produced M-CSF during monocyte differentiation with GM-CSF and IL-4. Thus, M-CSF could ultimately skew monocyte differentiation toward MΦ -like cells. Minor changes in AD-DC are also due to IL-6 (i.e. increase of CD14 and CD163 expression), which could reflect an endogenous production of this cytokine by DC at very low levels compared to SCC cells. However, other factors not analyzed here are responsible for most of the MΦ characteristics of AD-DC. Tumor-derived prostanoids present during monocyte differentiation has strong influence on the generation of DC able to modify the cytokine profile of naïve CD4+ T lymphocytes. Endogenously produced IL-10 by tumor cell-treated DC has effects mainly on cytokine production by DC and lymphocytes
Acknowledgments
The support of the Juarez Hospital Blood Bank staff for providing adult blood samples is gratefully acknowledged. We also thank Victor H. Rosales for help with flow cytometry, Héctor Vilchis, Julio C. Ramírez, and Juana Narváez for technical assistance, and Ms Ninfa Arreola for her aid in the preparation of the manuscript. This work was partially supported by a grant to C.S.T. from CONACYT (No. 42712). F.A.M. and S.B. are recipients of CONACYT pre-doctoral scholarship (Nos. 142632 and 144142, respectively).
Abbreviations
- AD
Adenocarcinoma
- APC
Antigen presenting cell
- CM
Conditioned media
- COX
cyclooxygenase
- Ctrl-DC
Control DC
- DC
Dendritic cell
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- IFN
Interferon
- IL
Interleukin
- LPS
Lipopolysaccharide
- mAb
Monoclonal antibody
- M-CSF
Macrophage colony-stimulating factor
- MΦ
Macrophage
- NSCLC
Non-small cell lung carcinoma
- PBMC
Peripheral blood mononuclear cells
- PG
Prostaglandin
- SCC
Squamous cell carcinoma
- SF
Soluble factors
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- TT
Tetanus toxoid
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;50:379. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 3.Niklinski J, NiklinskaW Laudanski J, Chyczewska E, Chyczewski L. Prognostic molecular markers in non-small cell lung cancer. Lung Cancer. 2001;34:S53. doi: 10.1016/S0169-5002(01)00345-2. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. International adjuvant lung cancer trial collaborative group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J Clin Oncol. 2004;22:2808. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 6.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755. [PubMed] [Google Scholar]
- 7.Kiertscher SM, Luo J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol. 2000;164:1269. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 8.Katsenelson NS, Shurin GV, Bykovskaia SN, Shogan J, Shurin MR. Human small cell lung carcinoma and carcinoid tumor regulate dendritic cell maturation and function. Mod Pathol. 2001;1:40. doi: 10.1038/modpathol.3880254. [DOI] [PubMed] [Google Scholar]
- 9.Makarenkova VP, Shurin GV, Tourkova IL, Balkir L, Pirtskhalaishvili G, Perez L, Gerein V, Siegfried JM, Shurin MR. Lung cancer-derived bombesin-like peptides down-regulate the generation and function of human dendritic cells. J Neuroimmunol. 2003;1–2:55. doi: 10.1016/j.jneuroim.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753. doi: 10.1016/S1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 11.Rotta G, Edwards EW, Sangaletti S, Bennett C, Ronzoni S, Colombo MP, Steinman RM, Randolph GJ, Rescigno M. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198:1253. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 13.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 15.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991. doi: 10.1038/sj.bjc.6690158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 17.Saji H, Nakamura H, Awut I, Kawasaki N, Hagiwara M, Ogata A, Hosaka M, Saijo T, Kato Y, Kato H. Significance of expression of TGF-beta in pulmonary metastasis in non-small cell lung cancer tissues. Ann Thorac Cardiovasc Surg. 2003;5:295. [PubMed] [Google Scholar]
- 18.Saha D, Pyo H, Choy H. COX-2 inhibitor as a radiation enhancer: new strategies for the treatment of lung cancer. Am J Clin Oncol. 2003;26:S70. doi: 10.1097/01.COC.0000074161.92815.93. [DOI] [PubMed] [Google Scholar]
- 19.De Vita F, Orditura M, Auriemma A, Infusino S, Roscigno A, Catalano G. Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep. 1998;5:649. [PubMed] [Google Scholar]
- 20.Songur N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H, Hizel N. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori. 2004;90:196. doi: 10.1177/030089160409000207. [DOI] [PubMed] [Google Scholar]
- 21.Yamaji H, Iizasa T, Koh E, Suzuki M, Otsuji M, Chang H, Motohashi S, Yokoi S, Hiroshima K, Tagawa M, Nakayama T, Fujisawa T. Correlation between interleukin 6 production and tumor proliferation in non-small cell lung cancer. Cancer Immunol Immunother. 2004;53:786. doi: 10.1007/s00262-004-0533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Torres C, Garcia-Romo GS, Cornejo-Cortes MA, Rivas-Carvalho A, Sanchez-Schmitz G. CD16+ and CD16- human blood monocyte subsets differentiate in vitro to dendritic cells with different abilities to stimulate CD4+ T cells. Int Immunol. 2001;13:1571. doi: 10.1093/intimm/13.12.1571. [DOI] [PubMed] [Google Scholar]
- 23.Iñiguez MA, Pablos JL, Carreira PE, Cabré F, Gomez-Reino JJ. Detection of COX-1 and COX-2 isoforms in synovial fluid cells from inflammatory joint diseases. Br J Rheumatol. 1998;37:773. doi: 10.1093/rheumatology/37.7.773. [DOI] [PubMed] [Google Scholar]
- 24.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Gonzalez JS, Aguilar-Cazares D, Prado-Garcia H, Nieto-Rodriguez A, Mandoki JJ, Avila-Moreno F, Rivera RM, Chavarria-Garces J. Lack of correlation between growth inhibition by TGF-beta and the percentage of cells expressing type II TGF-beta receptor in human non-small cell lung carcinoma cell lines. Lung Cancer. 2002;38:149. doi: 10.1016/S0169-5002(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 26.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 27.Mitani H, Katayama N, Araki H, Ohishi K, Kobayashi K, Suzuki H, Nishii K, Masuya M, Yasukawa K, Minami N, Shiku H. Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br J Haematol. 2000;109:288. doi: 10.1046/j.1365-2141.2000.02020.x. [DOI] [PubMed] [Google Scholar]
- 28.Rieser C, Ramoner R, Bock G, Deo YM, Holtl L, Bartsch G, Thurnher M. Human monocyte-derived dendritic cells produce macrophage colony-stimulating factor: enhancement of c-fms expression by interleukin-10. Eur J Immunol. 1998;28:2283. doi: 10.1002/(SICI)1521-4141(199808)28:08<2283::AID-IMMU2283>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-β1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567. [PubMed] [Google Scholar]
- 31.Sombroek CC, Stam AGM, Masterson AJ, Lougheed SM, Schakel MJAG, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168:4333. doi: 10.4049/jimmunol.168.9.4333. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard WC, Alley MC, McLemore TL, Boyd MR. Profiles of prostaglandin biosynthesis in sixteen established cell lines derived from human lung, colon, prostate, and ovarian tumors. Cancer Res. 1998;48:4770. [PubMed] [Google Scholar]
- 33.Sy SM, Wong N, Lee TW, Tse G, Mok TS, Fan B, Pang E, Johnson PJ, Yim A. Distinct patterns of genetic alterations in adenocarcinoma and squamous cell carcinoma of the lung. Eur J Cancer. 2004;40:1082. doi: 10.1016/j.ejca.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Kalinski P, Hilkens CMU, Snijders A, Snijdewint FGM, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naïve T helper cells. J Immunol. 1997;159:28. [PubMed] [Google Scholar]
- 35.Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM. Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res. 1998;58:1208. [PubMed] [Google Scholar]
- 36.Maihofner C, Charalambous MP, Bhambra U, Lightfoot T, Geisslinger G, Gooderham NJ, Colorectal Cancer Group. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis. 2003;24:665. doi: 10.1093/carcin/bgg006. [DOI] [PubMed] [Google Scholar]
- 37.Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, Chouaib S, Mami-Chouaib F. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small lung cancer patients. Int J Cancer. 1998;77:7. doi: 10.1002/(SICI)1097-0215(19980703)77:1<7::AID-IJC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Menetrier-Caux C, Thomachot MC, Alberti L, Montmain G, Blay JY. IL-4 prevents the blockade of dendritic cell differentiation induced by tumor cells. Cancer Res. 2001;61:3096. [PubMed] [Google Scholar]
- 39.Delneste Y, Charbonnier P, Herbault N, Magistrelli G, Caron G, Bonnefoy JY, Jeannin P. Interferon-γ switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 40.Whittaker DS, Bahjat KS, Moldawer LL, Clare-Salzler MJ. Autorregulation of human monocyte-derived dendritic cell maturation and IL-12 production by cyclooxygenase-2-mediated prostanoid production. J Immunol. 2000;165:4298. doi: 10.4049/jimmunol.165.8.4298. [DOI] [PubMed] [Google Scholar]
- 41.Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, Pontillo M, Mercalli A, Di Carlo V, Allavena P, Piemonti L. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10 high IL-12 low regulatory dendritic cell. J Immunol. 2004;172:7341. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 42.Harizi H, Norbert G. Inhibition of IL-6, TNF-α, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol. 2004;228:99. doi: 10.1016/j.cellimm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol. 2004;7:4630. doi: 10.4049/jimmunol.172.7.4630. [DOI] [PubMed] [Google Scholar]
- 44.Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]
- 45.Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J. 2004;18:1439. doi: 10.1096/fj.03-0969fje. [DOI] [PubMed] [Google Scholar]
- 46.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766. [PubMed] [Google Scholar]
- 47.Orsini E, Guarini A, Chiaretti S, Mauro FR, Foa R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003;63:4497. [PubMed] [Google Scholar]
- 48.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 49.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34+ progenitors by tumor cells: Role of Interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778. [PubMed] [Google Scholar]
- 50.Ohgami A, Tsuda T, Osaki T, Mitsudomi T, Morimoto Y, Higashi T, Yasumoto K. MUC1 mucin mRNA expression in stage I lung adenocarcinoma and its association with early recurrence. Ann Thorac Surg. 1999;67:810. doi: 10.1016/S0003-4975(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 51.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 52.Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage colony-stimulating factor are deficient in IL-12 production. Eur J Immunol. 1998;28:2498. doi: 10.1002/(SICI)1521-4141(199808)28:08<2498::AID-IMMU2498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 53.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the Interleukin-6 gene mediate induction by prostaglandins, cyclic AMP and lipopolysaccharide. Mol Cell Biol. 1994;14:4443. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YL, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]