Abstract
CD4+CD25+ regulatory T cells have been characterized as a critical population of immunosuppressive cells. They play a crucial role in cancer progression by inhibiting the effector function of CD4+ or CD8+ T lymphocytes. However, whether regulatory T lymphocytes that expand during tumor progression can modulate dendritic cell function is unclear. To address this issue, we have evaluated the inhibitory potential of CD4+CD25+ regulatory T cells from mice bearing a BCR–ABL+ leukemia on bone marrow-derived dendritic cells. We present data demonstrating that CD4+CD25+FoxP3+ regulatory T cells from tumor-bearing animals impede dendritic cell function by down-regulating the activation of the transcription factor NF-κB. The expression of the co-stimulatory molecules CD80, CD86 and CD40, the production of TNF-α, IL-12, and CCL5/RANTES by the suppressed DC is strongly down-regulated. The suppression mechanism requires TGF-β and IL-10 and is associated with induction of the Smad signaling pathway and activation of the STAT3 transcription factor.
Keywords: Tumor immunity, Tolerance, Dendritic cells, Regulatory T cells
Introduction
Regulatory T cells are comprised of a heterogeneous population of lymphocytes actively involved in modulating immune responses. They constitute key components of peripheral tolerance, regulating potentially autoreactive T cells that have escaped negative selection in the thymus. One such suppressive T cell population, the CD4+CD25+ subset, has become the focus of intensive research since its initial identification in autoimmune diseases [1, 2]. In the murine system, elimination of CD4+CD25+ regulatory T cells by early thymectomy results in the development of autoimmune diseases that can be suppressed by adoptive transfer of these cells [3]. Furthermore, autoimmune disorders induced by CD4+CD25− T lymphocyte infusion in lymphopenic mice could be abrogated by the co-transfer of CD4+CD25+ T cells [4].
Besides their role in autoimmunity, CD4+CD25+ regulatory T cells participate in the control of infection, transplantation tolerance and tumor immunity [1, 5, 6]. A mounting body of evidence indicates that CD4+CD25+ regulatory T cells contribute to the immune tolerance of cancer [1, 7–10]. An increase in the number of these cells has been detected in the blood, lymph nodes or spleens of tumor-bearing hosts [11–15], and their therapeutic depletion can result in improved responses to cancer immunotherapy [14–17].
The phenotype of these regulatory cells is defined by the expression of CD25, the α-chain of the IL-2 receptor, and other markers such as CTLA-4 (cytotoxic T lymphocyte-associated antigen 4), GITR (Glucocorticoid-induced TNF receptor), CD62L, LAG-3 or the toll-like receptors (TLR) [18]. However, all these cell surface molecules imperfectly distinguish CD4+CD25+ regulatory T cells from conventional, activated CD4+ T lymphocytes since both cell subsets express similar markers, and a clear molecular characterization of these suppressive cells is still pending. Recently identified, the X chromosome-encoded forkhead/winged helix transcription factor FoxP3 appears fundamental for the development and function of CD4+CD25+ regulatory T lymphocytes, and remains the most specific molecular marker for these cells [19–21]. The suppressive mechanisms induced by CD4+CD25+ regulatory T lymphocytes have not been completely elucidated. While the requirement for a direct cell to cell contact is well documented for regulatory T cells to trigger their immunosuppressive activity on their target cells, the role of inhibitory cytokines such as tumor growth factor β (TGF-β) and interleukine-10 (IL-10) is still being explored [2, 21–24].
Although the suppressive function of CD4+CD25+ regulatory T cells on effectors CD4+ and CD8+ T lymphocytes and B cells is well established, their effects on dendritic cells (DC), remain poorly defined [25–28]. More importantly, the actions of tumor-expanded regulatory T lymphocytes on dendritic cells are unknown. These effects may be critical since maintenance of DC in an immature or tolerogenic state by tumor-induced regulatory T cells may constitute an additional mechanism by which cancer cells evade the immune system. To address this issue, we have examined the immunosuppressive function of CD4+CD25+ regulatory T cells from tumor-bearing mice on bone marrow-derived dendritic cells (DC). Our results demonstrate that tumor-derived CD4+CD25+ regulatory T cells suppress DC maturation and function by down-regulating the activation of the transcription factor κB (NF-κB) in DC. The inhibitory mechanism requires a direct cell to cell contact, involves TGF-β and IL-10 and is associated with the induction of the Smad intracellular signaling pathway, and the activation of the signal transducer and activator of transcription 3 (STAT3) transcription factor. The inhibited DC produce lower levels of tumor necrosis factor α (TNF-α), IL-12, and CCL5/RANTES (chemokine (C–C motif) ligand 5/regulated upon activation normal T cell expressed and secreted) and lose their ability to stimulate allogeneic T cells. In addition, DC suppression can also be triggered in vivo by injection of tumor-derived CD4+CD25+ regulatory T cells. Our findings thus highlight DC as an additional potential target of tumor-induced regulatory T cells in the course of cancer progression.
Material and methods
Mice
Mice were housed under specific pathogen-free conditions and cared for according to the guidelines of the University of Arizona Institutional Animal Care and Use Committee. Female 6–8 weeks old BALB/c (H2d) and C57BL/6 mice (H2b) from the National Cancer Institute (Bethesda, MD, USA) were used for the experiments.
Cell line
The murine leukemia cell line 12B1 (kindly provided by Dr. W. Chen) was generated by retroviral transformation of BALB/c bone marrow cells with the human bcr–abl (b3a2) fusion gene [29, 30]. 12B1 cells express the p210 bcr–abl protein and when injected into BALB/c mice result in an aggressive leukemia, with the 100% lethal dose (LD100) being 102 cells after tail vein injection and 103 cells after subcutaneous injection [31]. The cells were cultured at 37°C, 5% CO2 in RPMI medium (Gibco/BRL, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gemini Bio-products, Woodland, CA, USA), 0.5 × minimal essential medium non-essential amino acids (Gibco/BRL) and 1 mM sodium pyruvate (Gibco/BRL). The 12B1 cell line was tested routinely and found to be free of Mycoplasma contamination.
Tumor generation
Female BALB/c mice were injected with 5×103 12B1 cells subcutaneously in the right groin and were monitored for tumor development. Tumor size was measured every other day once the tumors became palpable. Tumor volume was calculated using the formula: length × width2 × π/6.
Generation of bone marrow-derived DC
DC were generated from BALB/c bone marrow cells cultured in a complete RPMI medium (Gibco/BRL) containing 10% fetal bovine serum (Gemini Bio-products), murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ, USA) and Interleukine-4 (IL-4; Peprotech) at the concentration of 10 ng/ml. On day 4–5 non-adherent and loosely adherent cells were collected and used in further experiments. Flow cytometry analysis indicated that 60–70% of the cells expressed CD11c.
Cell purification by magnetic cell sorting and co-culture experiments
Total splenocytes were isolated from mice bearing established (2,000–3,000 mm3) 12B1 tumors. CD4+CD25+ T lymphocytes were purified by magnetic cell sorting using a mouse CD4+CD25+ T regulatory cell isolation kit and an autoMACSTM separator according to the manufacturers’ instructions (Miltenyi Biotec, Auburn, CA, USA). The purified T cells were then co-cultured with day 4 DC for 24 h (DC to CD4+CD25+ T lymphocytes ratio=1:1), and LPS (Sigma Chemical, St Louis, MO, USA) was added (1 μg/ml) for an additional 24 h. In some experiments, anti-TGFβ-1,2,3 (R & D Systems, Minneapoli, MN, USA), anti-IL-10 (R & D) blocking Ab or isotype controls were added at the beginning of the co-culture. In other experiments DC and CD4+CD25+ T lymphocytes were separated by a 0.4-μm pore size Transwell insert (Corning incorporated life sciences, Acton, MA, USA). CD11c+ cells were then purified from the co-cultures using CD11c+ microbeads and the autoMACSTM separator (Miltenyi Biotec) for further analysis. Positively and negatively selected cells were routinely analyzed by flow cytometry to assess the purity of each fraction.
Real-time PCR for FoxP3 expression
CD4+CD25+ T lymphocytes were purified as described above. Total RNA was extracted using TRIzol reagent (GibcoBRL). RNA was reverse transcribed using Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) and random primers. The cDNA was amplified by quantitative Real-time PCR using primers for Foxp3 or the 18S Ribosomal RNA housekeeping gene as followed: reactions were carried out in 96-well plates containing 10 μl of Bio-Rad iQ Supermix (Bio-Rad), 1 μl of Taqman primer/probe set, 2 μl of cDNA synthesis reaction, and 7 μl of molecular grade water. Reactions were run and analyzed on a Bio-Rad iCycler iQ Real-Time PCR detection system. The primers were synthesized by Applied Biosystems (Foster City, CA, USA). Normalized values for Foxp3 mRNA expression in each sample were calculated as the relative quantity of Foxp3 divided by the relative quantity of 18S ribosomal RNA.
Flow cytometry analysis and antibodies
DC, total splenocytes or purified CD4+CD25+ T cells were incubated for 5 min with an Fc receptor-blocking antibody (BD Biosciences Pharmingen, San Diego, CA, USA), then for 30 min with saturating amounts of the appropriate primary antibodies in PBS containing 3% heat-inactivated fetal bovine serum and 0.09% sodium azide (Sigma Chemical), then washed and analyzed using a FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). The following antibodies were used: FITC-anti-CD4, PE-anti-CD25, PerCP-anti-CD3, biotin-conjugated anti-TGFβ1 then streptavidin-FITC, PE-anti-CD11c, FITC-anti-I-Ad, FITC-anti-CD80, FITC-anti-CD86, and FITC-anti-CD40 (BD Biosciences Pharmingen); FITC-anti-CD62L, FITC-anti-CD152/CTLA-4, FITC-anti-GITR, FITC-anti-IL-10 (eBioscience, San Diego, CA, USA). Isotype control antibodies were obtained from BD Biosciences Pharmingen.
Mixed leukocyte reaction
Splenocytes from C57BL/6 mice (H2b) were treated for 20 min with 50 μg/ml mitomycin C (Sigma Chemical), washed and used as stimulators. Responder splenocytes (1×105) from naïve BALB/c mice (H2d) were plated in U-bottom 96-well plates. C57BL/6 splenocytes were added at a ratio of stimulators to responders of 1:5. CD4+CD25+ T cells purified from tumor-bearing BALB/c mice were then plated at a CD4+CD25+ T cell to responder splenocyte ratio of 1:1. After a 3-day co-culture, 1 μCi [3H]thymidine (ICN Pharmaceuticals, Costa Mesa, CA, USA) was added to each well. Cells were harvested 18 h later and the radioactiviy measured on a Packard beta counter (Packard Biosciences, Meriden, CT, USA). In some experiments, after the co-culture, the supernatants from each group were collected for the determination of cytokine production. In other experiments, the ability of bone marrow-derived DC (previously exposed to CD4+CD25+ T cells from tumor-bearing mice) to stimulate allogeneic T cells in mixed leukocyte reaction (MLR) was evaluated. CD11c+ DC were purified from the co-culture as described above, treated with mitomycin C (20 min, 50 μg/ml) and plated with responder C57BL/6 splenocytes at a DC to splenocyte ratio of 1:5. [3H]thymidine incorporation by the responder splenocytes was assessed as described above.
Effects of CD4+CD25+ T cells on DC in vivo
BALB/c mice (three per group) were injected subcutaneously with PBS, or LPS (10 μg per mouse), or CD4+CD25+ T lymphocytes (2×106) or received a co-injection of LPS (10 μg per mouse) and CD4+CD25+ T lymphocytes (2×106). After 2 days, the draining lymph nodes were removed, dissociated and CD11c+ cells were purified as described above. The cells were then treated with mitomycin C (20 min, 50 μg/ml) before being used as stimulators in MLR experiments. The responder cells were C57BL6 splenocytes (DC to splenocyte ratio of 1:5). [3H]thymidine incorporation by the responder splenocytes was assessed as described above.
Detection of cytokine and chemokine production by ELISA
Cell culture supernatants were collected, centrifuged to remove cell debris, frozen and stored at −80°C until further use. The concentration of the following cytokines was determined using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers’ instructions: IFN-γ, TNF-α, IL-12, IL-10 (eBiosciences) and TGF-β and CCL5/RANTES (R & D Systems).
Detection of I-κBα, Smad-2 and STAT3 phosphorylation by western blotting
Following the culture with CD4+CD25+ T cells, DC were purified and lysed in lysis buffer (1% Nonidet P40, 50 mM Tris–HCl, pH 7.4, 2 mM EDTA, 100 mM NaCl, 0.2 mg/ml Aprotinin, 0.2 mg/ml Leupeptin, 1 mM PMSF, 10 mM NaF, 30 mM NaPPi, 10 mM Na3VO4). Positive controls for I-κB phosphorylation consisted of DC treated with LPS (1 μg/ml). DC treated with recombinant mouse TGF-β1 (90 min, 20 pg/ml) or IL-10 (20 min, 10 ng/ml) were used as positive controls for Smad-2 or STAT3 phosphorylation, respectively. Negative controls consisted of DC cultured alone. Equal amount of proteins (30 μg) were analyzed by western blotting as described [32], using anti-phospho Smad-2, anti-Smad2, anti-phospho I-κBα, anti-I-κBα, anti-phospho STAT3 or anti-STAT3 antibodies (Cell Signalling, Beverly, MA, USA).
Detection of NF-κB and STAT3 activation by DNA-binding transcription factor ELISA assay
CD11c+ DC were purified following the co-culture with CD4+CD25+ T cells as described above. Nuclear extracts were performed using a Nuclear Extract kit (Active Motif, Carlsbad, CA, USA). Then NF-κB P50 or STAT3 DNA-binding activity was measured with 20 μg nuclear extract using the NF-κB P50 or STAT3 Trans-AMTM kit, respectively, according to the manufacturer’s recommendations (Active Motif, Carlsbad, CA, USA). Positive controls for STAT3 activation consisted of DC treated with IL-10 (20 min, 10 ng/ml); while DC treated with LPS (1 μg/ml) were used as the positive controls for NF-κB P50 activation.
Statistical analysis
The data depicted in each figure correspond to one representative experiment of at least three that were performed independently in each case. Student’s t tests were used to evaluate significance between groups.
Results
Characterization of CD4+CD25+ regulatory T cells in tumor-bearing mice
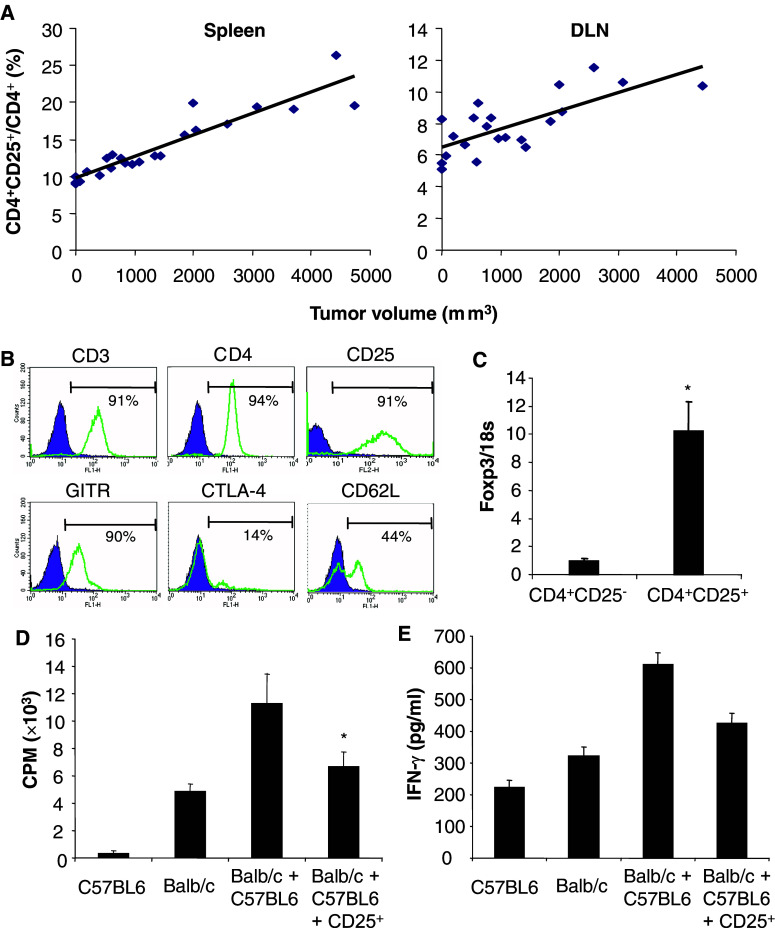
The expansion of CD4+CD25+ T cells with immunosuppressive properties during cancer progression has been described in humans and in several animal models. We first identified and characterized the phenotype and function of CD4+CD25+ T cells from tumor-bearing mice in the 12B1 BCR–ABL+ leukemia model. The ratio of CD4+CD25+/CD4+ in the spleen and in the draining lymph nodes increased in parallel with the growth of tumors (Fig. 1a). CD4+CD25+ T cells were isolated by magnetic cell sorting from the spleen of mice bearing established 12B1 tumors (2,000–3,000 mm3) and analysed by flow cytometry. The purified cells expressed characteristic surface markers identified on CD4+CD25+ regulatory T cells (Fig. 1b). Using real-time quantitative PCR, we confirmed that purified CD4+CD25+ T cells expressed high levels of the foxp3 transcript compared to their CD25− counterparts (Fig. 1c).
Fig. 1.
Characteristics of CD4+CD25+FoxP3+ regulatory T cells in mice bearing established 12B1 tumors. a Increase in the ratio of CD4+CD25+ to total CD4+ during tumor progression. Total splenocytes (Spleen) or draining lymph node (DLN) cells from mice bearing established 12B1 tumors at different stages were analyzed. The ratio CD4+CD25+/CD4+ was determined for each individual mouse and reported as a function of the tumor volume (r 2=0.9270, P<0.00005 in the spleen and r 2=0.7364, P<0.0005 in the draining lymph nodes). b Phenotype of CD4+CD25+ T lymphocytes purified using magnetic cell sorting from the spleen of mice bearing established 12B1 tumors (2,000–3,000 mm3). The percentage of positive cells is indicated. c Foxp3 mRNA levels are increased in CD4+CD25+ T cells purified from the spleen of mice bearing established tumors. Expression levels (average ± SD) relative to 18S ribosomal RNA are shown (*P<0.01). d Inhibition of the proliferation of allogeneic splenocytes by CD4+CD25+ T cells from tumor-bearing mice. Responder BALB/c splenocytes (Balb/c) were stimulated with C57BL6 splenocytes (C57BL6) in presence or absence of CD4+CD25+ T cells (CD25+) isolated from the spleen of mice bearing 12B1 tumors. The data are shown as mean ± SD of quadruplicate wells of 3[H]thymidine incorporation. e CD4+CD25+ T cells from tumor-bearing mice inhibit IFN-γ production. The culture supernatants of the experiment described in d were collected and IFN-γ concentration was determined by ELISA. The results are shown as the mean of duplicate wells. Asterisk is a significant difference when compared to control without CD4+CD25+ regulatory T cells (P<0.01)
The immunosuppressive function of CD4+CD25+ T cells isolated from tumor-bearing mice was observed in MLR inhibition assays. Purified CD4+CD25+ T lymphocytes suppressed the proliferation (Fig. 1d) and IFN-γ production (Fig. 1e) of responder splenocytes from naïve BALB/c mice stimulated by mitomycin C treated splenocytes from naïve C57BL/6 mice. Together, these results indicate that CD4+CD25+ T lymphocytes isolated from 12B1 tumor-bearing mice demonstrate specific features of immunosuppressive regulatory T cells.
CD4+CD25+ regulatory T cells from tumor-bearing mice suppress the expression of co-stimulatory molecules on DC
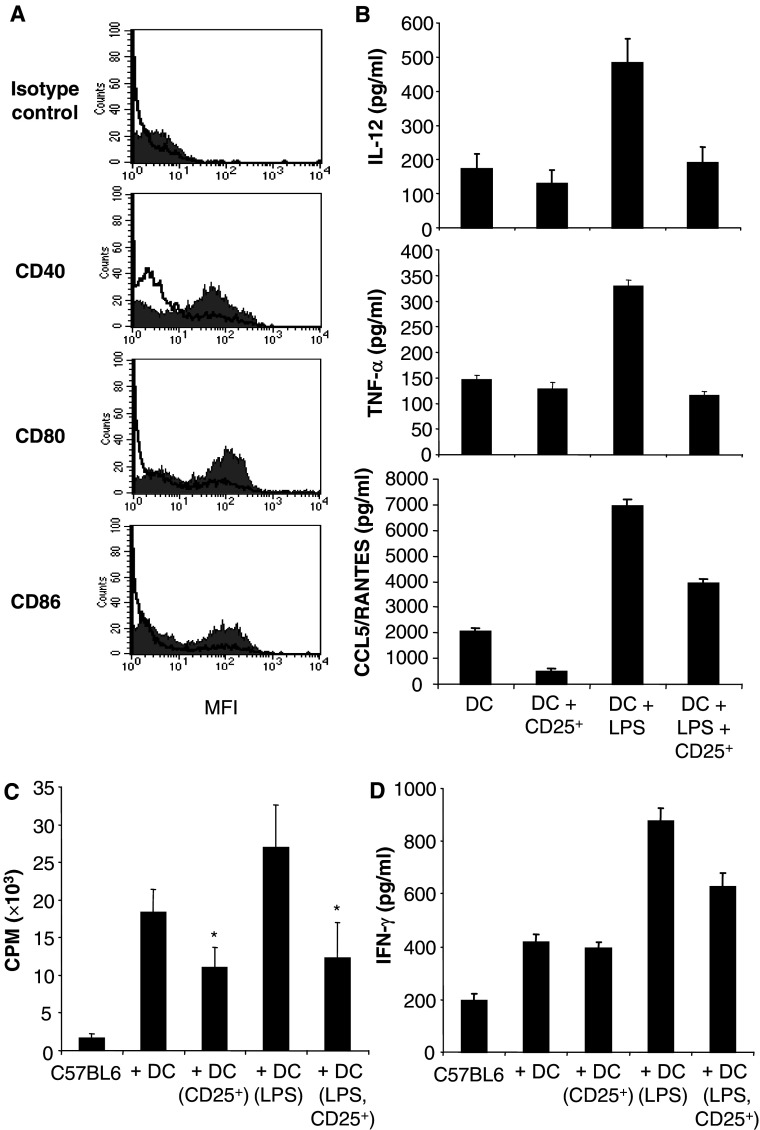
Mature DC expressing co-stimulatory molecules on their surface are capable of initiating anti-tumor immune responses by activating antigen specific CD4+ and CD8+ T lymphocytes. Therefore, we sought to examine whether CD4+CD25+ regulatory T cells from tumor-bearing hosts could dampen the expression of maturation markers on DC. Immature DC generated from the bone marrow were co-cultured for 24 h with regulatory T cells purified from the spleen of tumor-bearing mice. LPS was then added to induce co-stimulatory molecule expression. DC cultured with tumor-derived regulatory T cells showed a significant reduction in the expression of the co-stimulatory molecules CD40, CD80 and CD86 induced by LPS (Fig. 2a). This suggests that regulatory T cells from tumor-bearing mice are able to suppress LPS-induced DC maturation/activation.
Fig. 2.
CD4+CD25+ regulatory T cells from tumor-bearing mice suppress DC. a CD4+CD25+ regulatory T cells (CD25+) from tumor-bearing mice inhibit the expression of the co-stimulatory molecules by DC. Day 4 bone marrow-derived DC were cultured in the presence or absence of CD4+CD25+ regulatory T cells isolated from tumor-bearing mice (tumor volume = 2,000–3,000 mm3), and subjected to LPS treatment. DC were then purified, stained and analyzed by FACS. Shaded histogram, DC + LPS; non-shaded histogram, DC + LPS + CD25+. b CD4+CD25+ regulatory T cells inhibit cytokine production by DC. CD4+CD25+ regulatory T cells-DC co-culture supernatants were collected and the production of IL-12, TNF-α and CCL5/RANTES was analyzed by ELISA. Control DC were cultured in medium alone. Results are the mean ± SD of triplicate wells. c The capability of DC to induce the proliferation of allo-splenocytes is hampered by CD4+CD25+ regulatory T cells from tumor-bearing mice. DC purified from the co-culture with CD4+CD25+ regulatory T cells were used as stimulator in an allogeneic MLR. C57BL6 splenocytes were the responder cells and were cultured alone (C57BL6), with DC (+DC), with DC pre-incubated with regulatory T cells (+DC(CD25+)), or LPS (+DC(LPS)), or both (+DC(LPS,CD25+)). The data are shown as mean ± SD of quadruplicate wells of 3H-thymidine incorporation. d CD4+CD25+ regulatory T cells isolated from 12B1 tumor-bearing mice inhibit the potency of DC to induce IFN-γ production by allogeneic splenocytes. The same experiments as described in c were performed. At the end of the MLR, the supernatants were collected and ELISA were carried out. Results are the mean ± SD of duplicate wells. Asterisk is a significant difference when compared to the corresponding control without CD4+CD25+ regulatory T cells (P<0.01)
CD4+CD25+ regulatory T cells inhibit IL-12, TNF-α and CCL5/RANTES secretion by DC
Activated DC secrete cytokines such as IL-12 and TNF-α that play a crucial role in the stimulation of T cells. To further address the role of regulatory T cells from tumor-bearing hosts on DC activation, LPS-induced secretion of DC cytokines was analyzed by ELISA. As expected, high levels of IL-12 were detected in the supernatant of DC cultures after LPS stimulation. IL-12 production was strongly inhibited by the presence of CD4+CD25+ regulatory T cells. Similarly, the secretion of TNF-α by DC was significantly reduced (Fig. 2b). Moreover, the chemokine CCL5/RANTES induced by LPS was also suppressed by regulatory T cells from tumor-bearing mice (Fig. 2b).
CD4+CD25+ regulatory T cells inhibit the antigen presenting function of DC
We then investigated whether the antigen presenting function of DC could be affected by tumor-derived regulatory T cells. DC were cultured with regulatory T cells isolated from the spleen of tumor-bearing mice and were subjected to LPS treatment. CD11c positive DC were then purified using magnetic cell sorting and were subsequently cultured with splenocytes from allogeneic C57BL/6 mice. DC activated with LPS were potent stimulators of allogeneic T cell proliferation in MLR. In contrast, the capacity of DC pre-cultured with regulatory T cells to induce proliferation (Fig. 2c) and stimulate IFN-γ production (Fig. 2d) of allogeneic splenocytes was abrogated. Together these results indicate that CD4+CD25+ regulatory T cells from tumor-bearing hosts are capable of suppressing DC function.
Nuclear factor κb (NF-κb) activation is inhibited by CD4+CD25+ regulatory T lymphocytes
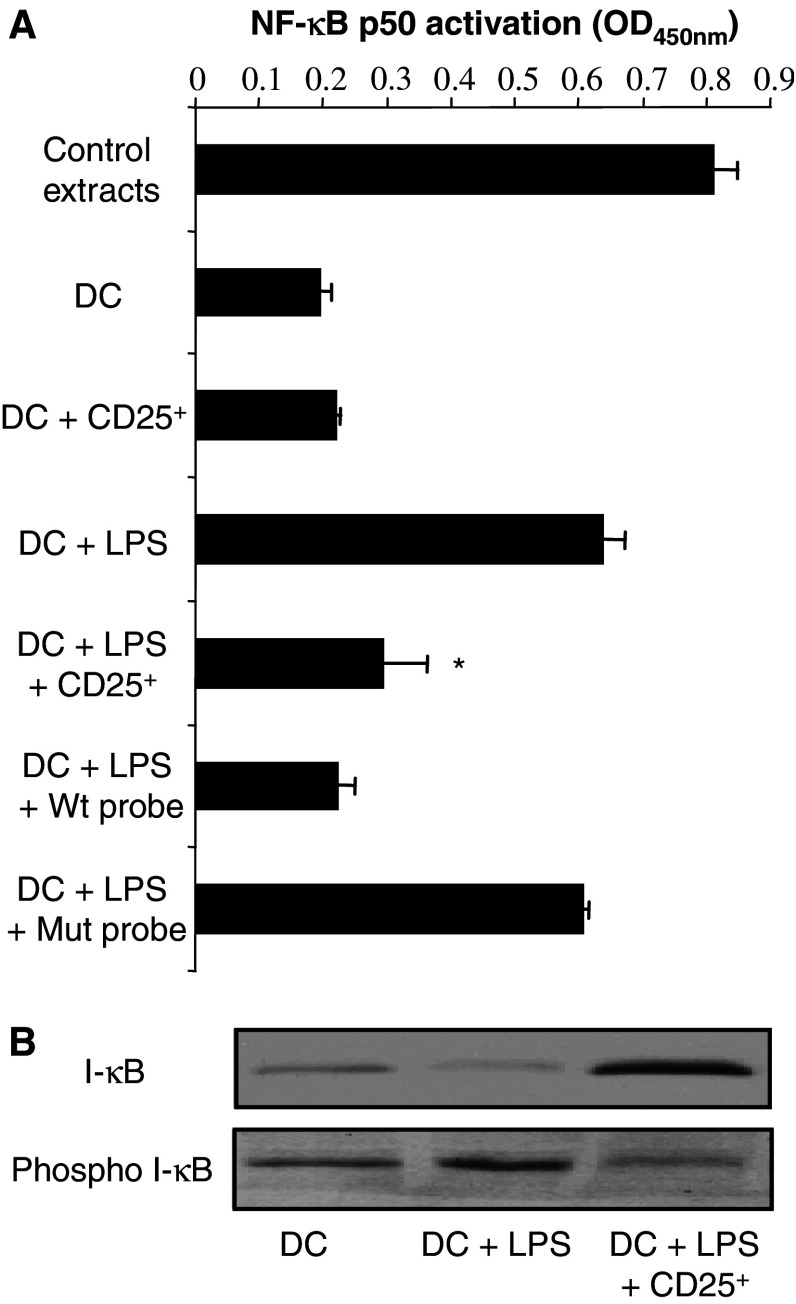
Nuclear factor κb (NF-κb) plays a central role in the activation of DC in response to various external stimuli or cytokines, and is involved in IL-12 and TNF-α secretion by these cells. NF-κb activation depends on the phosphorylation of its inhibitors, I-κB by Iκ-B kinase (IKK) leading to the translocation of the transcription factor in the nucleus where it binds to specific DNA promoter sequences. To gain further insight into the mechanism by which regulatory T cells from tumor-bearing hosts inhibit DC function, we examined their effects on NF-κb activation. DC were purified using CD11c microbeads from DC-regulatory T cell co-cultures, and nuclear extracts were prepared. The DNA binding activity of NF-κB to an immobilized oligonucleotide probe containing the consensus NF-κB site was then assessed using the TransAM kit technology. As expected, NF-κB P50 binding to its specific oligonucleotide probe was significantly increased in the nuclear extracts from DC activated with LPS compared to unstimulated DC. NF-κB P50 DNA binding activity was reduced in DC cultured in the presence of regulatory T cells from tumor-bearing mice (Fig. 3a). Competition experiments confirmed the specificity of NF-κB P50 binding (Fig. 3a). Consistent with these results, I-κB phosphorylation was detected by western blotting in cellular extracts from DC treated with LPS. In contrast, the presence of regulatory T cells significantly hampered the phosphorylation of I-κB induced by LPS in DC purified from the co-culture (Fig. 3b).
Fig. 3.
Inhibition of LPS-induced activation of nuclear factor-κB in DC by CD4+CD25+ regulatory T cells from tumor-bearing mice. a CD4+CD25+ regulatory T cells (CD25+) inhibit the DNA binding activity of NF-κB. CD11c+ cells were isolated at the end of the co-culture with or without CD4+CD25+ T lymphocytes, in the presence or absence of LPS. Nuclear extracts were performed and the DNA binding activity of NF-κB P50 to a consensus DNA oligonucleotide probe was assessed as described in Materials and methods. Positive controls (control extracts), provided by the manufacturer, correspond to TPA-treated Jurkat cells. To confirm DNA-binding specificity of the transcription factor, wild type (Wt) or mutated (Mut) NF-κB consensus oligonucleotides were added to the assay. The data are shown as mean ± SD of duplicate wells of NF-κB P50 activation determined as the OD value at 450 nm as indicated by the manufacturer. Asterisk is a significant difference when compared to the corresponding control without CD4+CD25+ regulatory T cells (P<0.01). b CD4+CD25+ regulatory T cells inhibit I-κB phosphorylation. Day 4 DC were cultured and CD11c+ cells were recovered as previously mentioned. Western blot analysis was performed for phospho-I-κB or I-κB. a, b Negative controls consisted of DC cultured alone, and positive controls of DC cultured with LPS (1 μg/ml)
DC suppression by CD4+CD25+ regulatory T cells requires direct cell to cell contact and involves TGF-β and IL-10
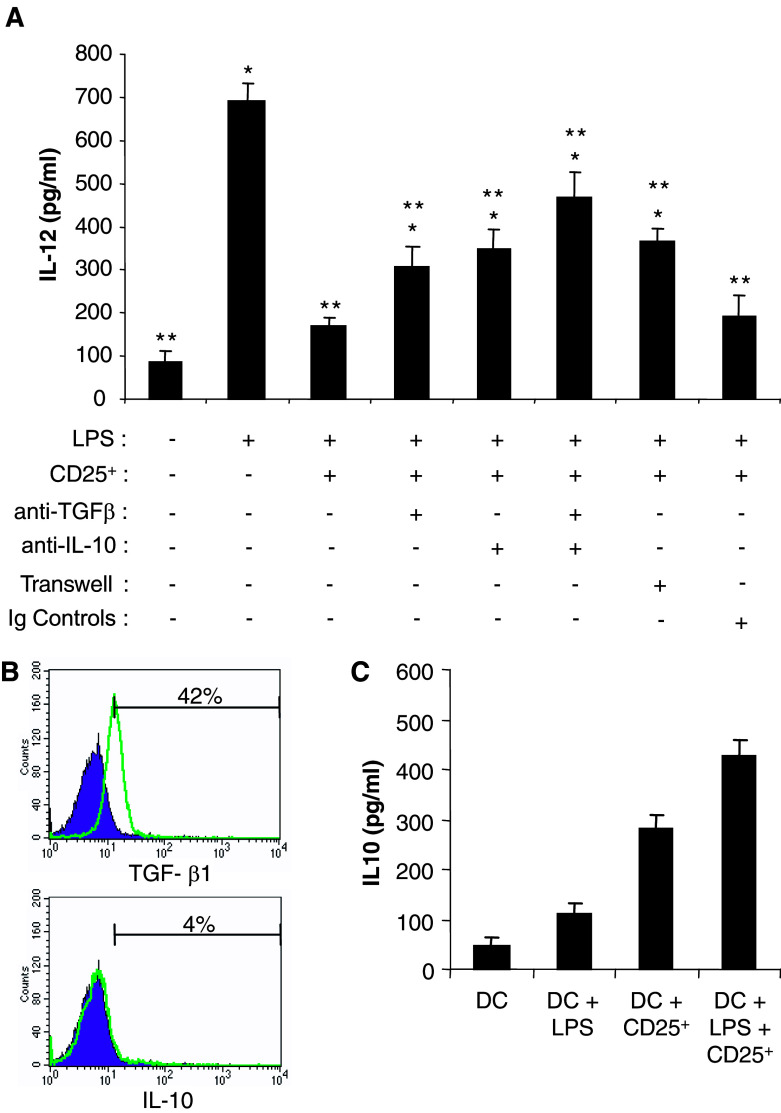
It is well established that CD4+CD25+ regulatory T cells exert their immunosuppressive effects on target cells in a contact-dependent manner. Transwell experiments were performed to examine whether the suppression of DC by regulatory T cells also requires direct cell to cell contact. The separation of the two cell types by a 0.4 μm pore size membrane compromised the suppressive activity of regulatory T cells since in these culture conditions the inhibition of IL-12 production was partially but significantly abrogated (Fig. 4a).
Fig. 4.
DC suppression by CD4+CD25+ regulatory T cells depends on TGF-β and IL-10. a The effects of CD4+CD25+ T cells (CD25+) are partially abrogated by anti-TGF-β1,2,3 or anti-IL-10 Ab. Day 4 DC were cultured with CD4+CD25+ regulatory T cells from tumor-bearing mice, with or without LPS, anti-TGF-β1,2,3, anti-IL-10 Ab, both anti-IL-10 and anti-TGF-β1,2,3 Ab, or isotype control antibodies as indicated. DC were also cultured separated from CD4+CD25+ T cells by a 0.4 μm pore size Transwell insert. IL-12 production was determined by ELISA in the culture supernatants. Results are the mean ± SD of duplicate wells. Asterisk is a significant difference when compared to LPS + CD4+CD25+ regulatory T cell group (P<0.05). Double asterisk is a significant difference when compared to LPS group (P<0.05). b Membrane expression of TGF-β1 and IL-10 by CD4+CD25+ regulatory T cells from tumor-bearing animal analyzed by flow cytometry. Representative histograms of three experiments are presented. Percentage of positive cells is indicated. c IL-10 secretion in the DC-CD4+CD25+ regulatory T cells co-cultures. Day 4 DC were cultured with CD4+CD25+ regulatory T cells and were treated with LPS as mentioned above. The indicated culture supernatants were tested for IL-10 concentration by ELISA. Results are the mean ± SD of duplicate wells
To further address the suppression mechanism induced by regulatory T cells in DC, the role of TGF-β and IL-10, two major cytokines reported to be involved in regulatory T cell suppressive activity, was examined. We first established that TGF-β1 was expressed at the cell membrane of regulatory T lymphocytes purified from the spleen of tumor-bearing mice (Fig. 4b). In addition, low but detectable levels of TGF-β1 were found in the supernatants of DC-regulatory T cell co-cultures (not shown). Anti-TGF-β1,2,3 blocking antibodies significantly reduced, but did not completely abrogate, the suppressive activity of regulatory T cells on IL-12 secretion by DC (Fig. 4a). IL-10 expression was not detected at the cell surface of regulatory T lymphocytes (Fig. 4b). Interestingly, the secretion of this cytokine was identified in the supernatants of DC cultured alone, but its concentration was higher in the co-cultures with regulatory T cells (Fig. 4c). In addition, anti-IL-10 blocking antibodies partially inhibited the suppressive activity of regulatory T cells on DC (Fig. 4a). Interestingly, the addition of both anti-TGF-β and anti-IL-10 antibodies did not completely reverse the inhibitory effects of regulatory T lymphocytes on DC production of IL-12 (Fig. 4a), indicating that other cytokines and/or mechanisms contribute to the immunosuppression of DC by regulatory T cells.
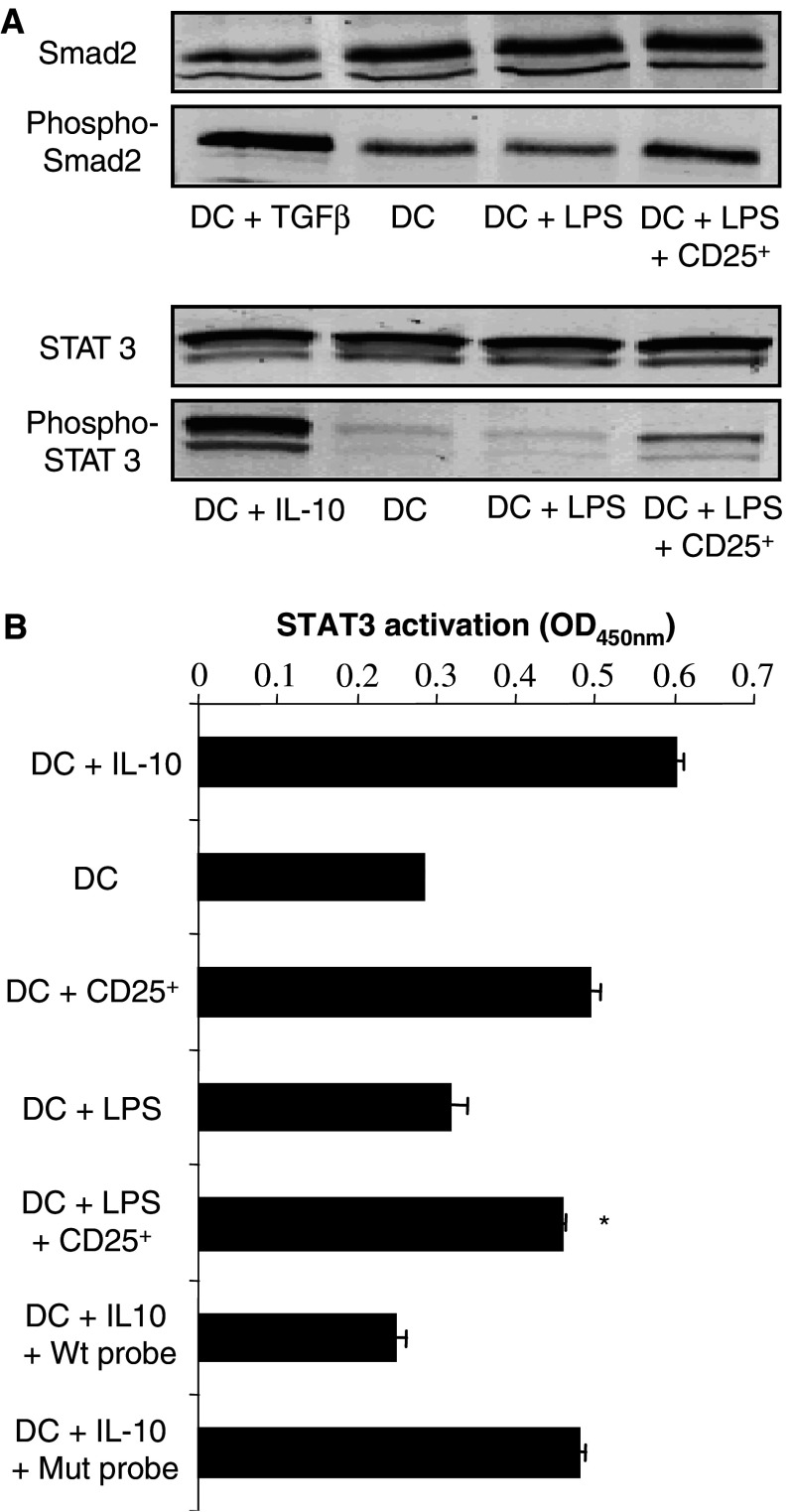
To provide further insight into the molecular mechanisms leading to DC inhibition, and since both IL-10 and TGF-β are involved, we sought to identify the intracellular signaling pathways induced within DC. TGF-β has been reported to trigger the Smad signaling cascade of events in target cells. Confirming our previous results, Smad2 phosphorylation was increased in cellular extracts of DC purified from the co-cultures with regulatory T cells, compared to cellular extracts of DC cultured alone or with LPS (Fig. 5a). The intracellular signaling machinery induced after IL-10 binding to its receptors causes the activation of the STAT3. Therefore, we examined whether regulatory T cells would induce STAT3 activation in DC. After culture alone or with LPS, a basal activation of STAT3 was detected using TransAM kits in DC nuclear extracts. The activation of STAT3 was however substantially increased after the co-culture with regulatory T cells (Fig. 5b). Consistently, the phosphorylation of STAT3 in DC was increased by CD4+CD25+ regulatory T cells (Fig. 5a).
Fig. 5.
DC suppression by CD4+CD25+ regulatory T cells is associated with the phosphorylation of Smad 2 and the activation of STAT3. a Detection of Smad2 and STAT3 phosphorylation in DC after co-culture with CD4+CD25+ regulatory T cells (CD25+). Day 4 DC were cultured in the presence or absence of CD4+CD25+ regulatory T cells, and treated with LPS. CD11c+ cells were selected and western blot analysis was carried out for phospho-Smad2, Smad2, phospho-STAT3, or STAT3. Positive controls for Smad2 or STAT3 phosphorylation consisted of DC treated with TGF-β (90 min, 20 pg/ml) or IL-10 (20 min, 10 ng/ml), respectively. Negative controls consisted of DC cultured alone. b CD4+CD25+ regulatory T cells enhance the DNA binding activity of STAT3 in DC. CD11c+ cells were obtained after co-culture with CD4+CD25+ regulatory T cells as outlined in (a). Nuclear extracts were performed and the DNA binding activity of STAT3 to a consensus DNA probe was assessed. To confirm the DNA-binding specificity of the transcription factor, wild type (Wt) or mutated (Mut) consensus oligonucleotides were added. Negative controls consisted of DC cultured alone, and positive control of DC treated with IL-10 (20 min, 10 ng/ml). The data are shown as mean ± SD of duplicate wells for which the OD value was determined at 450 nm as indicated by the manufacturer. Asterisk is a significant difference when compared to the corresponding control without CD4+CD25+ regulatory T cells (P<0.01)
Taken together these results indicate that regulatory T cells from tumor-bearing mice mediate DC suppression by a TGF-β and IL-10 dependent manner which is associated with the induction of the Smad signaling pathway, and the activation of the transcription factor STAT3, respectively.
Activated DC are not susceptible to CD4+CD25+ regulatory T cell inhibition
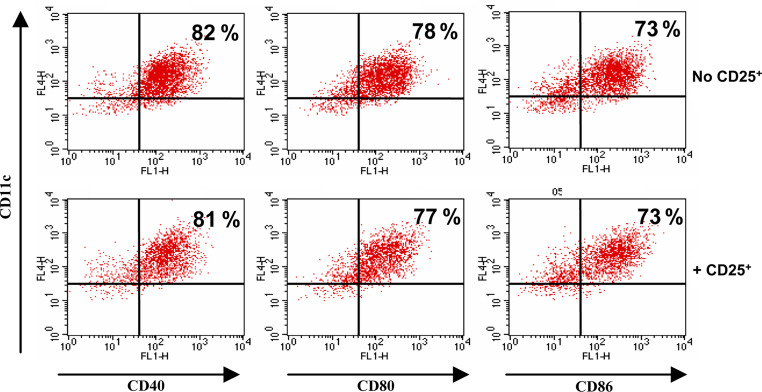
Since regulatory T cells from tumor-bearing hosts are capable of inhibiting the activation of immature DC by LPS, we investigated whether these suppressive cells could also hamper mature DC function. Bone marrow-derived DC were first activated with LPS to express CD80, CD86 and CD40 and to secrete high levels of IL-12 (not shown). These activated DC were then cultured in the presence of regulatory T cells from tumor-bearing mice. The expression of CD80, CD86, CD40 (Fig. 6), and the secretion of IL-12 or TNF-α (not shown) were not down-regulated by regulatory T cells, indicating that after full differentiation and maturation, DC become insensitive to the suppressive effects of CD4+CD25+ regulatory T cells.
Fig. 6.
Activated DC are not susceptible to CD4+CD25+ regulatory T cell-mediated suppression. Day 4 DC were first activated with LPS (1 μg/ml) and then co-cultured (+CD25+) or not (No CD25+) with CD4+CD25+ regulatory T cells from tumor-bearing animals. CD11c+ cells were then purified, stained for the indicated markers and analyzed by flow cytometry. Percentage of positive cells is indicated
CD4+CD25+ regulatory T cells from tumor-bearing mice suppress DC function in vivo
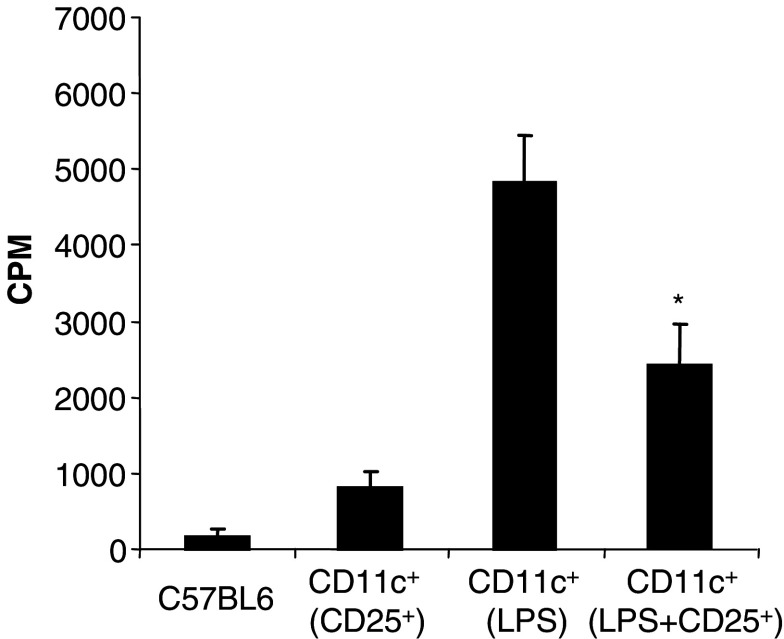
To address whether regulatory T cells from tumor-bearing hosts are also capable of inhibiting DC in vivo, healthy naïve mice were injected subcutaneously with PBS, or LPS, or CD4+CD25+ regulatory T lymphocytes or both LPS and regulatory T cells. CD11c+ cells isolated from the draining lymph nodes of LPS-treated mice were more effective allo-stimulators compared to DC from PBS or CD4+CD25+ T cells-treated animals. The co-injection of CD4+CD25+ T cells with LPS demonstrated a significant inhibitory effect on DC harvested from the lymph nodes of treated animals (Fig. 7). This indicates that CD4+CD25+ T lymphocytes obtained from tumor-bearing hosts can also modulate DC function in vivo following adoptive transfer into syngeneic healthy mice.
Fig. 7.
CD4+CD25+ regulatory T lymphocytes from tumor-bearing mice modulate DC function in vivo. Mice (three per group) received PBS, or LPS (10 μg), or CD4+CD25+ T cells isolated from tumor-bearing mice (2×106 cells) or LPS and CD4+CD25+ T cells injection (sc). Two days later, the draining lymph nodes were collected and CD11c+ cells were positively selected, treated with mitomycin C and used as stimulators in a MLR, with C57BL6 splenocytes (C57BL6) as responders. The data are shown as mean ± SD of triplicate wells of 3[H]thymidine incorporation. Asterisk is a significant difference when compared to the corresponding control without CD4+CD25+ regulatory T cells (P<0.01)
Discussion
CD4+CD25+FoxP3+ T lymphocytes are the center of multiple immunoregulatory processes in various disease states including cancer. In tumor-bearing hosts, their depletion can augment anti-tumor immune responses [7, 14, 16, 17], however the mechanisms of suppression remain incompletely elucidated. Previous studies have documented that these cells compromise the function of anti-tumor effector CD8+ T cells and can also hamper CD4+ T cell help [7, 9, 15, 33, 34]. However, the immunosuppressive effects of regulatory T cells from tumor-bearing hosts on DC have not been previously reported. Our current results indicate that CD4+CD25+FoxP3+ T lymphocytes obtained from mice bearing an aggressive BCR–ABL+ leukemia have potent inhibitory actions on bone marrow-derived DC such as suppression of CD40, CD80 and CD86 expression induced by LPS. Moreover, regulatory T cells from tumor-bearing mice inhibit the production of the pro-inflammatory cytokines IL-12 and TNF-α, hamper the secretion of the chemoattractant chemokine CCL5/RANTES, and suppress DC capacity to induce the activation of T lymphocytes. Importantly, these immunosuppressive effects are associated with the inhibition of the transcription factor NF-κB. Consistent with our results, previous reports have indicated that IL-12 and TNF-α production by DC is under the control of this transcription factor [35]. Interestingly, the expression of CD80, CD86, CD40 and the secretion of IL-12 and TNF-α by DC first matured with LPS and then cultured with regulatory T cells were not down-regulated, indicating that DC after full differentiation and maturation become insensitive to the suppressive effects of CD4+CD25+ regulatory T cells.
Many of the mechanisms of regulatory T cell-mediated suppression remain conflicting and controversial. Direct cell–cell interaction between regulatory T cells and their target cells has been established as a prerequisite for the triggering of regulatory T cell effects [36]. However, the role of TGF-β and IL-10 has been the subject of extensive investigation leading to divergent results. Thus, it has been reported that the suppression of CD25− T lymphocyte proliferation by regulatory T cells does not require TGF-β [37]. In addition, it has been shown that the proliferation of CD4+CD25− T cells from TGF-βRII-dominant negative transgenic mice and Smad3-deficient mice can be inhibited by CD4+CD25+ T cells from wild type mice. In the same study CD4+CD25+ T lymphocytes from neonatal TGF-β1−/− mice were capable of inhibiting effector T cells [24]. In contrast, other studies demonstrated that CD4+CD25+ T lymphocytes produced TGF-β and IL-10, expressed TGF-β1 at the cell surface and mediated immunosuppression of T and B cells in a TGF-β dependent manner [22, 23]. In the current study, we found that tumor-derived regulatory T cell suppression of DC requires direct CD4+CD25+ T cell-DC contact and can be partially reversed by anti-TGF-β1,2,3 or by anti-IL-10 antibodies. Since only low levels of soluble TGF-β have been detected in the co-cultures, it is possible that the actions of TGF-β on DC merely depend on the membrane form detected on CD4+CD25+ T cells. Interestingly, the presence of both anti-TGF-β1,2,3 and anti-IL-10 antibodies does not completely reverse regulatory T cell-induced suppression, which suggests that although these two cytokines contribute to the immunosuppressive effects of regulatory T cells, other factors also appear to play a role.
The intracellular cell signaling induced by TGF-β involves the phosphorylation of the cytosolic Smad proteins including Smad2 and Smad3. These molecules associate to form a hetero-oligomeric complex with Smad4 that translocates to the nucleus where it regulates the transcription of specific genes by binding directly to DNA consensus sequences, or by interacting with other transcription factors [38]. Consistently, DC incubated with regulatory T cells isolated from tumor-bearing mice exhibited increased levels of Smad2 phosphorylation. IL-10 suppresses the production of pro-inflammatory cytokines including TNF-α and IL-12, and chemokines such as MIP1α or RANTES by triggering the phosphorylation and heterodimerization of the STAT3 transcription factor [39, 40]. Our results indicate that the culture of DC with CD4+CD25+ regulatory T cells results in the activation of STAT3 in DC. Since STAT3 has been reported to modulate NF-κB binding to specific promoter DNA sequences [41], these results provide a link between IL-10-dependent inhibition of DC by regulatory T lymphocytes and the down-regulation of NF-κB observed in the suppressed DC.
It has been previously documented that CD4+CD25+ T cells from healthy naïve mice can down-regulate DC co-stimulatory molecule (CD80 and CD86) expression [27], and IL-12 secretion [26]. CD4+CD25+ T lymphocytes from healthy human donors exhibit suppressive effects on monocyte/macrophages [42] and on DC generated from peripheral blood monocytes [25]. However, these previous studies did not explore the effects of regulatory T cells from tumor-bearing hosts, nor did they investigate the molecular mechanisms of suppression or the intracellular signaling events induced within DC as outlined herein. In addition the effects of regulatory T cells on DC in vivo had not been determined. Since regulatory T cells have been reported to expand during tumor progression and to play a key role in inducing tolerance to tumors, it was essential to determine whether they can inhibit the first steps of the immune response at the DC level. A profound deficit in the function of DC (lack of co-stimulatory molecule expression, decreased secretion of pro-inflammatory cytokines, inability to activate T lymphocytes) has been reported in cancer patients and in various animal tumor models that may account for tumor escape from the immune system [43–47]. Our results indicate that regulatory T cells from tumor-bearing mice are capable of maintaining bone marrow-derived DC in an immature/tolerogenic state. These so-called tolerogenic DCs are believed to induce T cell anergy [43, 46–49], and may also be involved in the generation of regulatory T cells [49–51]. Such a positive feed-back loop by which tolerogenic DC induce regulatory T cell that in turn enhance their immune inhibitory function could contribute to the establishment and persistence of tumor-induced tolerance. The inhibition by regulatory T lymphocytes of the chemokine CCL5/RANTES secretion by DC that we observed here constitutes an additional factor that could further obstruct the induction of specific anti-tumor immune responses, by suppressing the recruitment of effector T lymphocytes or inflammatory cells.
Thus, our results highlight DC as a susceptible strategic target for CD4+CD25+ regulatory T cells from tumor-bearing hosts, and provide additional mechanisms triggered by tumors to escape immune surveillance. Since numerous anti-tumor vaccination strategies are based on the use of DC as APCs, these findings further emphasize the need of eliminating CD4+CD25+ T lymphocytes in cancer immunotherapy protocols.
Acknowledgments
The authors wish to thank Pawel Kiela for his helpful comments and Jennifer Uno for her help with real-time PCR. This work was supported in part by the NIH grant R01 CA104926 and the Tee Up for Tots Fund.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25(+)CD4(+) regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 3.Asano M, Toda M, Sakaguchi N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Belkaid Y, Piccirillo CA, Mendez S, et al. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 6.Cobbold SP, Nolan KF, Graca L, et al. Regulatory T cells and dendritic cells in transplantation tolerance: molecular markers and mechanisms. Immunol Rev. 2003;196:109–124. doi: 10.1046/j.1600-065X.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 8.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 9.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo EY, Yeh H, Chu CS, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 11.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 12.Ormandy LA, Hillemann T, Wedemeyer H, et al. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 13.Liu JY, Zhang XS, Ding Y, et al. The changes of CD4+CD25+/CD4+ proportion in spleen of tumor-bearing BALB/c mice. J Transl Med. 2005;3:5. doi: 10.1186/1479-5876-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 15.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad SJ, Farrand KJ, Matthews SA, et al. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot JD, Rasmussen JP, Williams LM, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Kitani A, Fuss I, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccirillo CA, Letterio JJ, Thornton AM, et al. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misra N, Bayry J, Lacroix-Desmazes S, et al. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Tateishi S, Kubo K, et al. Downregulation of IL-12 and a novel negative feedback system mediated by CD25(+)CD4(+) T cells. Biochem Biophys Res Commun. 2005;330:226–232. doi: 10.1016/j.bbrc.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 27.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Serra P, Amrani A, Yamanouchi J, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/S1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Qin H, Reese VA, et al. CTLs specific for bcr–abl joining region segment peptides fail to lyse leukemia cells expressing p210 bcr–abl protein. J Immunother. 1998;21:257–268. doi: 10.1097/00002371-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin J, Chianese E, Witte ON. In vitro transformation of immature hematopoietic cells by the P210 BCR/ABL oncogene product of the Philadelphia chromosome. Proc Natl Acad Sci USA. 1987;84:6558–6562. doi: 10.1073/pnas.84.18.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He L, Feng H, Raymond A, et al. Dendritic-cell-peptide immunization provides immunoprotection against bcr–abl-positive leukemia in mice. Cancer Immunol Immunother. 2001;50:31–40. doi: 10.1007/PL00006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng H, Zeng Y, Whitesell L, et al. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood. 2001;97:3505–3512. doi: 10.1182/blood.V97.11.3505. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa H, Jager E, Ritter G, et al. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 34.Somasundaram R, Jacob L, Swoboda R, et al. Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res. 2002;62:5267–5272. [PubMed] [Google Scholar]
- 35.Onishi H, Kuroki H, Matsumoto K, et al. Monocyte-derived dendritic cells that capture dead tumor cells secrete IL-12 and TNF-alpha through IL-12/TNF-alpha/NF-kappaB autocrine loop. Cancer Immunol Immunother. 2004;53:1093–1100. doi: 10.1007/s00262-004-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 37.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Wahl SM. TGF-beta: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85–89. doi: 10.1016/S1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 39.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 40.Williams LM, Ricchetti G, Sarma U, et al. Interleukin-10 suppression of myeloid cell activation—a continuing puzzle. Immunology. 2004;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoentjen F, Sartor RB, Ozaki M, et al. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- 42.Taams LS, van Amelsfort JM, Tiemessen MM, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 44.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 45.Chaux P, Favre N, Martin M, et al. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int J Cancer. 1997;72:619–624. doi: 10.1002/(SICI)1097-0215(19970807)72:4<619::AID-IJC12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 47.Monti P, Leone BE, Zerbi A, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10 high IL-12 low regulatory dendritic cell. J Immunol. 2004;172:7341–7349. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 48.Steinbrink K, Jonuleit H, Muller G, et al. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 49.Kuwana M. Induction of anergic and regulatory T cells by plasmacytoid dendritic cells and other dendritic cell subsets. Hum Immunol. 2002;63:1156–1163. doi: 10.1016/S0198-8859(02)00754-1. [DOI] [PubMed] [Google Scholar]
- 50.Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T Cells. J Immunol. 2005;174:7433–7439. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 51.Mahnke K, Qian Y, Knop J, et al. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]