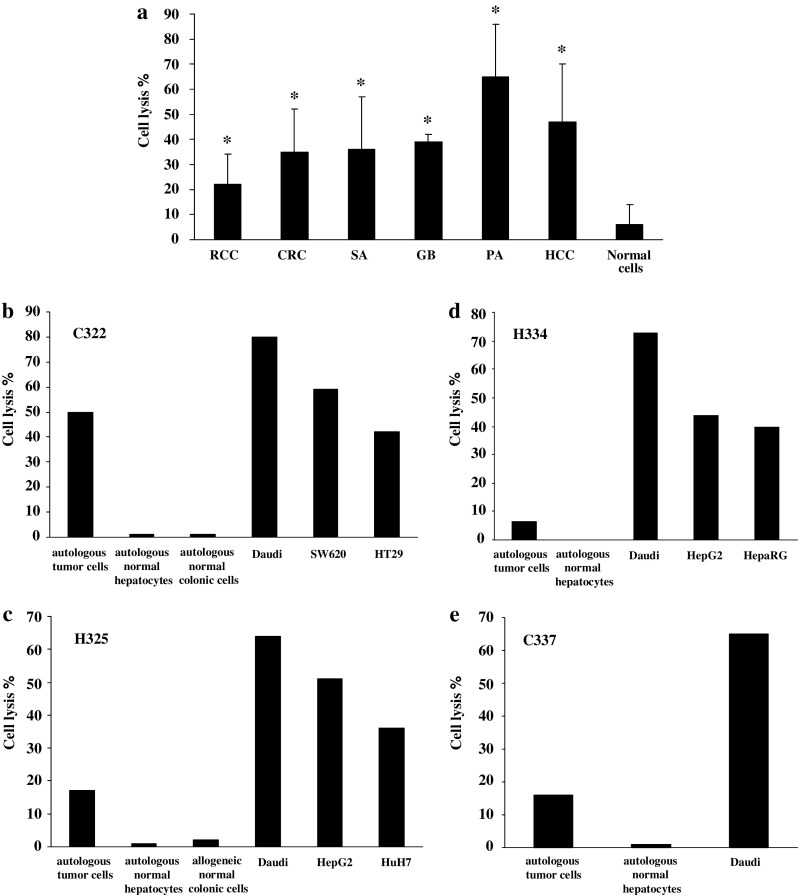
Fig. 3.
Cytotoxic activity of Vγ9Vδ2 T cells from cancer patients against tumor cells from different origins. PBMCs were treated at day 0 with 300 nM BrHPP and cultured in presence of 400 IU IL-2/ml within 2 weeks. Expanded Vγ9Vδ2 T cells were incubated for 4 h with 5 × 103 target cells previously labeled with 51Cr in a ratio effector to target (E/T) of 50/1. Results are the mean of assays performed in triplicate. *: significant difference when compared with cytotoxicity against normal target cells. a Cytotoxic activity in allogeneic context. PBMCs were from seven patients suffering from CRC, HCC or sarcoma. Target cells were RCC (R104, R119, R131 cell lines and R180, R305 primary cultures) (n = 8, P = 0.01), CRC (C181, C187, SW620, SW403, HT29 cell lines, and C293 primary culture) (n = 22, P = 0.001), HCC (HepG2, HuH7, and BC2 cell lines) (n = 8, P = 0.002), sarcoma (SA297, SA309 primary cultures) (n = 4, P = 0.048), glioblastoma (GB2, GB3 primary cultures) (n = 4, P = 0.004), and tumoral pancreatic cells (PA287 primary cultures) (n = 2, P = 0.044). Normal cells used as control targets were normal colonic (n = 2) and normal hepatic cells (n = 2) from patients and PBMCs from healthy donors (n = 8). b–e Selective cytotoxic activity against autologous tumor cells. Target cells were autologous normal and tumor cells isolated from liver or colonic biopsies from patient C322 (b), H325 (c), H334 (d), and C337 (e). Cytotoxicity against normal allogeneic cells and CRC (SW620, HT29), HCC (HepG2, HuH7, and HepaRG), and Daudi cell lines was also evaluated