Abstract
Background
Breast cancer patients frequently harbour tumour-reactive memory T cells in their bone marrow (BM) but not in the blood. After reactivation ex-vivo these cells rejected autologous breast tumours in xenotransplanted mice demonstrating therapeutic potential upon reactivation and mobilization into the blood. We conducted a clinical pilot study on metastasized breast cancer patients to investigate if ex-vivo reactivation of tumour-reactive BM memory T cells and their adoptive transfer is feasible and increases the frequencies of tumour-reactive T cells in the blood.
Methods
The study protocol involved one transfusion of T cells which were reactivated in vitro with autologous dendritic cells pulsed with lysate of MCF7 breast cancer cells as source of tumour antigens. Immunomonitoring included characterization of T cell activation in vitro and of tumour-specific T cells in the blood by interferon (IFN)-γ ELISPOT assay, HLA-tetramers and antigen-induced interleukin (IL)-4 secretion.
Results
Twelve patients with pre-existing tumour-reactive BM memory T cells were included into the study. In all cases, the treatment was feasible and well tolerated. Six patients (responders) showed by ELISPOT assay de-novo tumour antigen-specific, IFN-γ-secreting T cells in the blood after 7 days. In contrast, non responders showed in the blood tumour antigen-induced IL-4 responses. All responders received more than 6.5 × 103 tumour-reactive T cells. In contrast, all non responders received lower numbers of tumour antigen-reactive T cells. This was associated with reduced activation of memory T cells in activation cultures, increased amounts of CD4+ CD25high regulatory T cells in the BM and increased tumour antigen-dependent IL-10 secretion. The latter was prevented by preceding depletion of regulatory T cells suggesting that regulatory T cells in the BM can inhibit reactivation of tumour-specific T cells.
Conclusion
Taken together, adoptive transfer of ex-vivo re-stimulated tumour-reactive memory T cells from BM of metastasized breast cancer patients can induce the presence of tumour antigen-reactive type-1 T cells in the peripheral blood.
Keywords: Breast cancer, Memory T cells, Adoptive T cell therapy
Introduction
Breast cancer is characterized by high numbers of recurrences that are due to an early dissemination of tumour cells. A predominant organ for their dissemination and maintenance is the bone marrow (BM) [1]. Current immunotherapies target residual tumour cells by tumour antigen-specific cytotoxic T lymphocytes (CTLs) generated through dendritic cell (DC) vaccinations, in vitro expansion of tumour antigen-specific CTL clones or adoptive transfer of T cell receptor-transduced T cells. While the latter approach needs to overcome major regulatory barriers and awaits verification of efficacy, clinical responses of the other two approaches varied between only 10 and 15% of the cases [2]. We here explore a third strategy that is based on the exploitation of the patients’ natural repertoire of tumour antigen-reactive memory T cells (TC).
The BM is capable of recruitment of antigen-loaded DC from peripheral sites, priming of T cells against local and systemic antigens and generation and recruitment of memory T cells [3–6]. In mice dormant BM-resident tumour cells caused the generation and maintenance of tumour antigen-specific memory T cells [7, 8] which prevented outgrowth of distant metastases [8]. Survival and competence of memory CD8 T cells in vivo depends on co-activated CD4 T helper cells [9–11]. Interferon (IFN)-γ secreted by TH1 cells directly suppresses tumour-progression by influencing the tumour stroma [12] and regulatory T cells (Treg) in situ [13].
An immunological relevance of the BM in breast cancer was proposed after the detection of tumour antigen-reactive cytotoxic and type-1 memory T cells at high frequencies in the BM [14–16]. In breast cancer, approximately 30% of tumour-specific memory T cells of the BM belong to the subpopulation of central-memory T cells that accumulate selectively in secondary lymphoid organs and represent a prominent T cell subpopulation in BM [3, 16]. Upon re-stimulation central memory T cells exert a particular high proliferative potential [17–19] and sustained generation of secondary effector T cells [19, 20] and mediated long lasting tumour regressions in mouse models [21, 22].
Tumour antigen-reactive memory T cells have been detected mainly in BM but not blood of breast cancer patients [14–16]. We hypothesized that insufficient reactivation of these BM resident T cells may contribute to their absence in the blood since it was shown that T cell reactivation in lymphoid organs precedes profound changes in expressed homing receptors which is followed by their emigration into the blood and subsequent infiltration into inflamed tissues [23]. Thus, specific reactivation of BM resident T cells from breast cancer patients in vitro might result in their acquisition of protective capacity in vivo. Indeed, upon reactivation in vitro with tumour antigen-pulsed autologous DC and adoptive transfer, BM-derived memory T cells from breast cancer patients rejected efficiently autologous breast tumours in NOD/Scid mice [15, 16]. Thus, under favourable circumstances of short term reactivation ex-vivo these T cells acquired anti-tumour effector capacities.
We therefore performed a clinical pilot study in metastasized breast cancer patients to evaluate the feasibility of an adoptive T cell transfer approach based on the aspiration, ex vivo reactivation and subsequent intravenous application of tumour-reactive CD4 and CD8 BM T cells and to evaluate if such approach increases the numbers of functional tumour antigen-reactive T cells in the blood.
First we tested for the presence of tumour-reactive memory T cells in BM of the patients by short term IFN-γ ELISPOT-analysis and subsequently treated positive patients by a single transfer of their own BM-derived T cells that were reactivated against breast cancer associated antigens derived from the breast cancer cell line MCF7.
We demonstrate that isolation, reactivation and adoptive transfer of BM T cells was feasible and well tolerated. Immunological efficacy by means of increased frequencies of activated type-1 tumour-reactive T cells in the peripheral blood was found in 50% of the patients, was associated with effective memory T cell reactivation in vitro and correlated with a minimal estimated number of approximately 6.5 × 103 transfused tumour-reactive T cells. No immune response was found in patients receiving less than 6.5 × 103 tumour-reactive T cells. In these patients, predominant tumour antigen-dependent TH2 profiles were detected in the blood after treatment. This was associated with low rates of memory T cell reactivation, increased proportions of Treg and increased interleukin (IL)-10 secretion during therapeutic T cell stimulation in vitro.
Methods
Patients
Blood and BM samples were taken from 70 18- to 75-year-old patients with histologically approved metastasized breast carcinomas without second malignancy, autoimmune disease, renal or heart failures or pregnancy after informed consent. The study protocol was approved by the institution’s ethical committee. BM (40 ml for diagnostic and 100 ml for therapeutic purposes) was aspirated under local anaesthesia from both iliac crests and 80 ml blood was collected by venipuncture. BM and blood samples were subjected to Ficoll gradient [15] and mononuclear cells were collected.
Generation of dendritic cells
Dendritic cells were generated as described [15]. For diagnostic purposes BM cells were cultured for 14 days in serum-free X-VIVO20 medium with 50 ng/ml rhuGM-CSF and 1,000 U/ml IL-4. B cells and T cells were removed by magnetic beads labelled with mAbs against CD19 and CD3 (Dynal, Oslo, Norway). Therapeutic DC were generated in 7 days from plastic-adherent mononuclear cells as described above. DC were pulsed for 20 h with cell lysates (200 μg protein/1 × 106 cells/ml) or for 3 h with 10 μg/ml of HLA-A2-restricted nonameric peptides in cytokine-free X-VIVO-20.
Antigens
As source of tumour antigens we generated lysate of the breast cancer cell line MCF7 [24] as described [15]. Cell fragments were removed by 30 min centrifugation at 13,000 rpm. The supernatant was tested for protein content and stored at −80°C. As source of control antigen, lysate of the promonocytic tumour cell line U937 was generated likewise. In some cases, HLA-A2+ DC were pulsed with HLA-A2-restricted peptides derived from breast cancer-associated antigens musin 1 (MUC1)12–20 [15, 16], Her-2/neu 369–377 [15, 16] or heparanase183–191 [25].
T cell culture
Mononuclear cells were cultured as described [15] in RPMI-1640 with 10% AB serum, 100 U/ml IL-2 and 60 U/ml IL-4. For therapeutic purposes, T cells from patients #6–12 were additionally substituted with 20 ng/ml IL-7 and IL-15 (Promocell, Heidelberg, Germany). Generally, T cells obtained from therapeutic BM aspirates were exclusively used for adoptive transfer (despite small aliquots required for sterility tests). Before stimulation T cells were purified using magnetic beads labelled with mAb against CD19, CD15 and CD56 (Dynal) and kept in cytokine-free RPMI-1640 with 10% AB serum. In some cases regulatory T cells (Treg) were depleted by CD4 negative and subsequent (CD4)CD25 positive selection using the CD4+ CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotech, Bergisch Gladbach, Germany), following the manufacturer’s instructions.
ELISPOT assays
Interferon-γ secreting T cells were determined using IFN-γ ELISPOT kits (Mabtech, Hamburg, Germany) and automated quantitation by ELISPOT reader as described [15]. Antigen-pulsed DC were co-incubated with autologous T cells (DC/T cell ratio = 1:5) for 40 h in triplicate wells. Cytokine spots measured in the presence of DC pulsed with control antigen were considered as background. Individuals were designated as responders if the numbers of spots in wells containing tumour antigen-loaded DC were significantly higher than in control wells by Student’s T-test (P ≤ 0.05). Frequencies of tumour-reactive T cells were calculated only in case of significant tumour antigen reactivity as follows: (spots in wells with TA-pulsed DC-spots in negative control wells) ÷ TC/well. In case that HLA-A2-restricted tumour antigen-derived peptides were used as test antigens a HLA-A2-restricted synthetic insulin34–42-derived peptide HLVEALY LV [16] was used as negative control antigen.
Flow cytometry
Mononuclear BM cells were treated with human Ig (Endobulin; Immuno, Heidelberg, Germany) to block unspecific binding sites. Treg were detected by staining of the cells with the following directly conjugated mouse mAbs against human antigens: FITC-CD4 (clone RPA-T4, BD Biosciences, Heidelberg, Germany), PE-Cy5-CD3 (HIT3a; BD Biosciences) and PE-CD25 (clone 4E3; Miltenyi Biotech, Bergisch Gladbach, Germany). For additional detection of FOXP3 expression, cells were subsequently fixed and permeabilized with Fix/Perm Buffer (eBioscience, USA), blocked with rat-serum and subsequently stained with FOXP3-APC. For detection of activated CD4 or CD8 memory T cells we used the mouse anti human mAbs FITC-CD4 (clone RPA-T4), FITC-CD8 (clone HIT8a), PE-CD45RO (clone UCHL-1), APC-CD69 (clone L78), all from BD Biosciences. Dead cells were labelled with propidium iodide (PI). Recordings were made only on PI negative cells. For T-cell staining with HLA-A2/peptide tetramers bone-marrow T cells from HLA-A0201+ patients were incubated for 10 min on ice with PE-conjugated Tetrameric complexes containing HLA-A0201 and MUC1-derived peptide M1.2 ([12–20]; LLLLTVLTV) [15], and FITC-CD8 monoclonal antibodies. HLA-A2 expression was analysed using FITC-labelled anti HLA-A2 mAb (clone BB7.2; BD Biosciences). FACSCalibur with Cell-Quest software (BD Biosciences, Heidelberg, Germany) was used for multicolour flow cytometry measurement. FlowJo software (Tree Star, San Carlon, CA, USA) was used for analysis of at least 20,000 events.
Dendritic cells were stained with a mixture of PE-labelled lineage marker specific mAbs against CD3, CD14, CD19 and CD56 (BD-Bioscience, Heidelberg, Germany), anti human CD11c-APC and FITC-labelled mAbs against human HLA-DR, CD80, CD40 or CD83. Dead cells were labelled with PI. Expression of HLA-DR, CD80, CD40 and CD86 was assessed on gated CD11c+, lineage marker-, PI-viable DCs. HLA-A2 expression was analysed on PBMCs using FITC-labelled anti HLA-A2 mAb (clone BB7.2; BD Biosciences).
Quantification of soluble factors
After 48 or 72 h of T cell stimulation supernatants from ELISPOT-test wells (48 h) or from therapeutic stimulation cultures (72 h) were collected and tested for presence of cytokines IL-4 and IL-10 using HS Immunoassay Quantikine® (R&D Systems, Wiesbaden, Germany). Supernatants from unstimulated, cytokine-free DC and TC cultures were collected after DC and TC generation over 72 h and analysed likewise.
CA15-3 concentrations in sera of breast cancer patients were routinely monitored through the central laboratory of the University Hospital of Heidelberg.
T cell stimulation and adoptive cell transfer
Purified T cells were coincubated with autologous MCF7 lysate-pulsed DC for 72 h (DC/TC ratio between 1:5 and 1:13) at 1 × 106 TC/ml medium (RPMI + 10% AB-serum). After 48 h half of the medium was substituted. After 72 h all cells in suspensions were washed, passed through a single cell filter, suspended in 100 ml 0.9% NaCl solution and transfused i.v. over 15 min. Aliquots were collected before and after stimulation for microbial testing. Adoptive transfer was performed when microbial contamination was undetectable after 3 days of blood culture by the institution’s Microbiology Department. All patients received antibiotic prophylaxis with i.v. administered ciprofloxacin (3 × 400 mg Ciprobay; Bayer, Leverkusen, Germany) and fluconazole (2 × 400 mg i.v. Diflucan®; Pfizer, Karlsruhe, Germany) for one day. Treatment was applied at the intensive care unit where the patients were observed for 24 h.
Microbial tests
Cell suspensions were subjected for 5 days to BACTEC Plus/F-hemoculture flasks (BD Biosciences) supplemented with BACTEC-Fos-Supplement (BD Biosciences). Microbial contamination was evaluated after 3 and 5 days by the Institution’s Microbiology Department.
In stimulation culture #10 contamination with very low numbers of Staphylococcus aureus was detected after 4 days of culture. The strain was sensitive to the antibiotic prophylaxis and no clinical or laboratory signs of infection, including repeated blood cultures was detected in the patient. All other cultures were sterile.
Toxicity assessment
Blood parameters (complete blood count, calcium, potassium, sodium, creatinine, urea nitrogen, GGT, GOT, gamma-GT, parameters of coagulation, c-reactive Protein) were assessed before and 24 h after the treatment. Furthermore, clinical examinations were done over a period of 24 h at the intensive care unit including skin inspection, neurological observation, measurement of blood pressure, pulse, body temperature and ECG. All patients were asked to report on any adverse symptoms during the whole follow-up period.
Results
Study design and feasibility
We included 18- to 75-year-old patients with metastasized breast cancer who contained tumour-reactive memory TC in their BM. All of the study patients had previously received standard cytostatic treatments such as chemotherapy or hormone therapy (Table 1) and were in a palliative treatment situation. Patients with secondary malignancies, auto-immune diseases, renal failure or pregnancy were excluded.
Table 1.
Clinical and experimental data
| Pat. no. | HLA-A2 | Disease state | Bone metastases | Serum CAl 5-3 before ADI | Pre-treatment | Tumour-reactive TC before ADI (per 106) | BM (ml) | Stimulated TC in culture (×107) | Transferred TC (×107) | TC/DC ratio during stimulation | Total TA-reactive TC in culture a(×103) | Type 1 TC response | Type 2 TC response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat. 01 | Pos. | SD | Neg. | Normal | AC, Tx, Ta, AI | 180 | 100 | 4.0 | 4.0 | 5:1 | 7.2 | Pos. | Neg. |
| Pat. 02 | Neg. | PD | Pos. | Normal | Ta, AI | 290 | 66 | 0.5 | 0.3 | 5:1 | 1.5 | Neg. | |
| Pat. 03 | Pos. | SD | Neg. | Normal | AC, Tx, Ta, AI | 120 | 96 | 2.0 | 1.0 | 5:1 | 0.2 | Neg. | Pos. |
| Pat. 04 | Neg. | PD | Pos. | Normal | Ta, AI | 220 | 35 | 0.5 | 0.2 | 13:1 | 1.1 | Neg. | Pos. |
| Pat. 05 | Neg. | SD | Neg. | Normal | AC, Tx, Ta, AI | 340 | 90 | 5.0 | 1.0 | 6:1 | 17.0 | Pos. | Neg. |
| Pat. 06 | Neg. | SD | Neg. | Normal | Ta | 460 | 108 | 3.5 | 2.0 | 7:1 | 16.1 | Pos. | Neg. |
| Pat. 07 | Neg. | PD | Pos. | Normal | Ta, AI | 200 | 105 | 2.5 | 2.5 | 5:1 | 5.0 | Neg. | |
| Pat. 08 | Pos. | PD | Pos. | Elevated | AC, Tx, Ta, AI | 540 | 68 | 0.6 | 0.3 | 6:1 | 3.2 | Neg. | Pos. |
| Pat. 09 | Pos. | SD | Neg. | Normal | AC, Tx, Ta, AI | 730 | 100 | 4.5 | 2.2 | 5:1 | 32.9 | Pos. | |
| Pat. 10 | Neg. | PD | Pos. | Elevated | Tx, AI | 470 | 104 | 1.3 | 0.9 | 5:1 | 6.1 | Neg. | |
| Pat. 11 | Neg. | PD | Neg. | Elevated | AC, Tx, Ta, AI | 640 | 107 | 6.2 | 5.7 | 8:1 | 39.7 | Pos. | |
| Pat. 12 | Neg. | PD | Neg. | Elevated | AC, Tx, Ta, AI | 680 | 100 | 1.0 | 0.4 | 5:1 | 6.8 | Pos. | |
| Correlation to T1 response | n.s., Fi, P = 0.49 | n.s., Fi, P = 0.11 | Neg., Fi, P = 0.01 | n.s., Fi, P = 0.46 | n.s., Fi, P = 0.12 | n.s., Fi, P = 0.28, T-test, P = 0.10 | n.s., Fi, P = 0.12, T-test, P = 0.09 | Pos., Fi, P = 0.04, T-test, P = 0.006 | Pos., Fi, P = 0.04, T-test, P = 0.09 | n.s., Fi, P = 0.5, T-test, P = 0.7 | Pos., Fi, P = 0.001, T-test, P = 0.01 | Neg., Fi, P = 0.05 |
Fi Fisher’s exact test; used for comparison of non-quantitative data or according to median value for each parameter in case of quantitative data. Quantitative data were also compared by two-sided Student’s T-test (T-test), SD stable disease, PD progressive disease, n.s. not significant, AC adriamycine/cyclophosphamide, AI, aromatase inhibitors, Ta tamoxifene, Tx taxol
aCalculated by multiplication of TA-reactive TC before ADI with numbers of stimulated TC
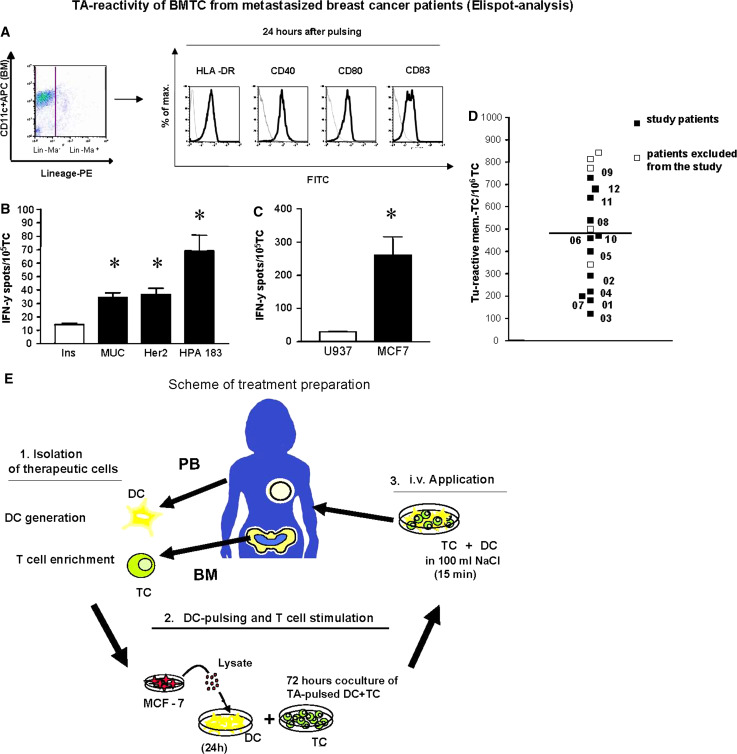
To test memory T cells from breast cancer patients, we performed short-term in vitro stimulation with common breast cancer tumour antigens presented by autologous DC to elicit secondary but not primary immune responses. The IFN-γ ELISPOT assay served as a read-out system to quantify T-cell responses at the cellular level. We stimulated BM T lymphocytes with DC pulsed with breast cancer tumour antigens derived from the allogeneic breast cancer cell line MCF-7. We have previously shown that upon cognate interactions with tumour-specific memory T cells DC from breast cancer patients receive maturation signals via CD40 and that tumour-specific memory T cells thereby do not require interactions with fully activated DC for appropriate activation ([15, 16, 25], Fig. 1a). Throughout the study we therefore did not further stimulate antigen-pulsed DC before their co-culture with T cells. We considered patients to be immunologically responsive when the numbers of spots in experimental wells were significantly higher (P < 0.05) compared with the negative control, that is, wells containing the same T cells and DC pulsed with lysate from promonocytic leukaemia cell line U937 as a source of irrelevant antigens.
Fig. 1.
Tumour antigen-reactivity of bone marrow T cells from metastasized breast cancer patients. a Activated DC phenotype after pulsing with tumour cell lysate. Generated DC were pulsed with tumour lysate for 24 h and phenotyped by flowcytometry. Left dot plot CD11c expression of gated, viable (PI-) lineage marker-DC. Right panel Expression of HLA-Dr, CD40, CD80 and CD83 on gated DCs (black lines). Isotype controls are shown as grey lines. b, c IFN-γ ELISPOT assay of bone marrow T cell from one HLA-A0201+ patient (who was not included into the study) with reactivity against HLA-A2-restricted peptides from the tumour antigens MUC1, Her-2/neu and heparanase (b) and tumour antigens derived from MCF-7 breast cancer cells (c). Mean values and SD of triplicate wells containing tumour antigens (black bars) or control antigens (b irrelevant control peptide HLVEALY LV, c U937-lysate, white bars) are shown. Asterisk Significant difference between test and respective control wells P < 0.05, Student’s T-test). d Frequencies of MCF-7-specific type-1 T cells in bone marrow of breast cancer patients as determined by IFN-γ-ELISPOT-analysis (P < 0.05 compared to negative control wells containing U937 lysate, Student’s T-test). Black squares patients who were included into the study, white squares patients excluded from study treatment. The numbers (Px) indicate different study patients. e Scheme of treatment preparation
Bone marrow from ELISPOT-positive patients was collected and T cells were enriched by depletion of contaminating cells. DC were generated from blood-derived monocytes in vitro by culture with GM-CSF and IL-4 according to standard protocols [15]. Then, DC were pulsed with tumour antigen and used to stimulate the BM T cells for 3 days. Finally, the co-cultured cells were intravenously applied as schematically shown in Fig. 1e.
We chose the well defined breast cancer cell line MCF7 cells as source of tumour antigens in order to broadly stimulate the highly polyvalent repertoire of tumour antigen-reactive CD4 and CD8 T cells described for breast cancer patients [26]. MCF7 cells express defined breast cancer antigens, such as MUC1 and Her-2/neu [27]. Therefore, MCF7 cells are used in clinical vaccination protocols for breast cancer patients [28]. We previously showed that transfer of MCF7 reactivated patient-derived BM memory T cells into breast cancer-bearing NOD/SCID mice mediated tumour rejection [15, 16]. In our hands, more than 80% of patients who contained in the BM T cells specific for autologous breast tumour antigens (autologous tumour cell lysates) also showed specific T cell reactivity against MCF7-derived tumour antigens, suggesting a significant overlap of tumour antigens from individual breast tumours and MCF7 [24, 27 and data not shown]. Figure 1b, c show reactivity of BM-derived T cells from one exemplary HLA-A0201 positive breast cancer patient against HLA-A0201-restricted peptides from breast cancer-associated antigens MUC112–20, Her-2/neu369–377 and heparanase183–191 [15, 16, 29] (b) and against MCF-7 lysate (c). To address if the ex vivo stimulation of BM-derived T cells with MCF7-derived antigens in our study caused the activation of T cells specific for defined breast cancer-associated antigens, we evaluated for each patient the expression of HLA-A0201 and then quantified the frequencies of MUC1-specific CD8 T cells in the blood of respective patients by HLA-tetramer analysis (Table 1).
Of 17 patients who fulfilled the inclusion criteria (Fig. 1d) two died before inclusion while another three refused inclusion due to their poor clinical condition. The remaining 12 patients were included in the study. The mean frequency of MCF7-specific T cells, as calculated by subtracting numbers of spots in negative control wells from numbers of spots in test wells, was 470/106 T cells.
MCF7-pulsed DC were used for stimulation of purified autologous BM T cells for 72 h at DC/T cell ratios between 1:5 and 1:13. Thereafter the cells were suspended in 100 ml 0.9% NaCl solution and transfused over 15 min under intensive care conditions (Fig. 1e). The patients were released into ambulant observation 24 h after T cell transfer.
Table 1 summarizes the frequencies of tumour-reactive T cells, the proportions of T cells, DC/T cell ratios and the numbers of transferred T cells for each patient together with their disease status. In all cases generation and enrichment of DC and T cells was possible although the numbers of transferred T cells highly varied between 2 × 106 and 5.7 × 107 per patient due to different amounts of BM obtained and different proportions of T cells in the BM. All study patients received the T cell transfer. None of the patients showed clinical or laboratory signs of treatment related toxicity. Especially allergic reactions and signs of nephrotoxicity were not observed. In two patients we observed a moderate flush 12 h after vaccination in combination with a slightly elevated body temperature of 37.5°C at most. Before treatment, five patients had stable disease while seven patients had progressive disease. At a first clinical follow up after 6 months eight patients had stable disease, two patients had progressive disease and two patients had died.
Immunological effects
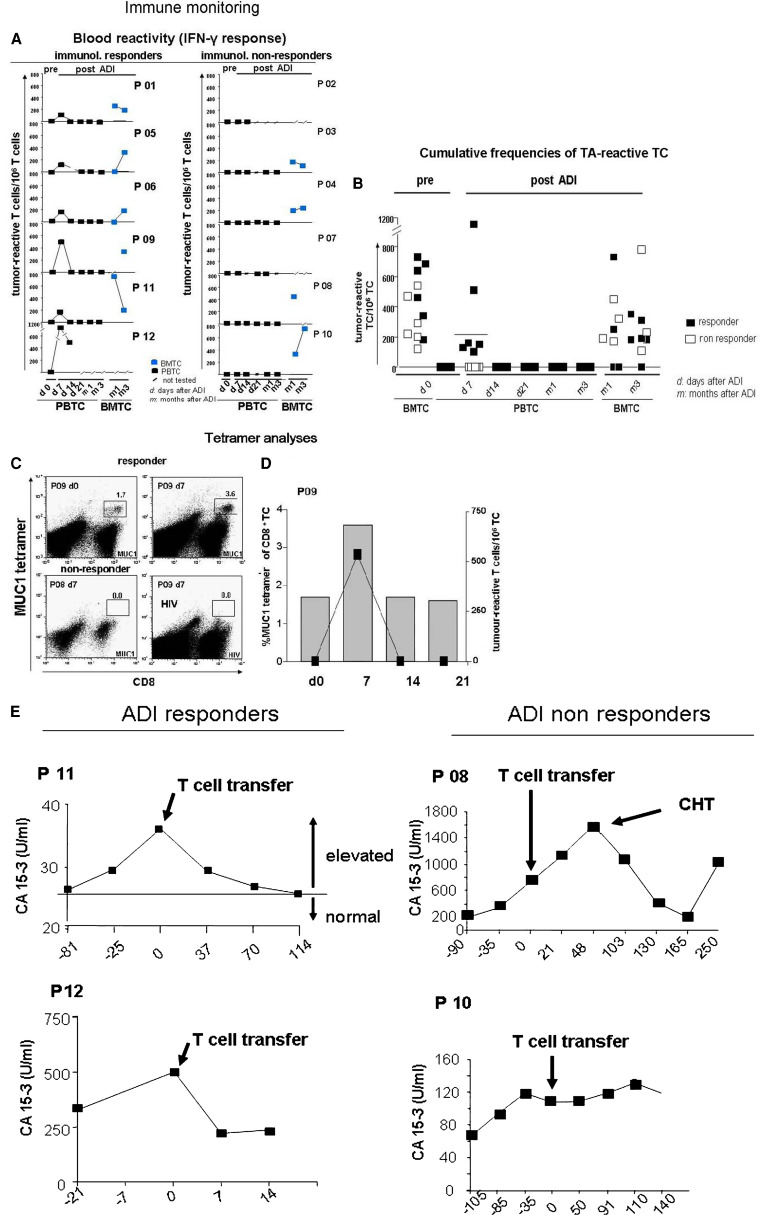
We collected peripheral blood prior to treatment, weekly during the first month and 12 weeks after treatment. BM was collected after 4 and 12 weeks. All samples were cryopreserved and simultaneously analysed. The results are shown in Fig. 2. Before T cell transfer, none of the patients contained MCF7-reactive T cells in their blood. This is in accordance with observations demonstrating the absence of functional tumour antigen-specific T cells in the blood of breast cancer patients [15]. One week after the transfer we observed in the blood from 6 out of 12 patients by IFN-γ-ELISPOT assay the presence of tumour antigen (MCF-7)-specific T cells. The mean frequency of tumour antigen-reactive T cells in all study patients after 1 week was 207/106 T cells (Fig. 2a, b), that of ELISPOT-positive patients was 414/106 T cells. These were not detectable at later time points. In the following, we refer to the latter group as (immunological) responders or adoptive immunotherapy (ADI) responders. Since the other six patients showed no tumour-reactive T cells in the circulation at any tested time point we refer to them as (immunological or ADI) non-responders. Our analyses revealed no significant impact of the T cell transfer on the numbers of tumour-reactive T cells in the BM of responders or non responders (Fig. 2a, b).
Fig. 2.
Immune monitoring. a, b Frequencies of MCF-7-specific type-1 T cells in the blood and bone marrow of treated patients prior to (pre) and after (post) adoptive immunotherapy (ADI) as determined by IFN-γ ELISPOT-analysis. Non-significant results are depicted at the bottom line. a Individual results from all study patients. b cumulative results. c, d Frequencies of MUC1- or HIV (as negative control antigen) -specific CD8 T cells among total mononuclear cells in the blood of one HLA-A0201+ responder (P09, c, d) and one HLA-A0201+ non responder (P07, c) as determined by flowcytometry through binding of MUC1 or HIV peptide- loaded-HLA-A2 tetramers before and after adoptive T cell transfer. A concomitant increase in the frequencies of MCF-7-specific type-1 T cells of P09 as determined by IFN-γ ELISPOT assay (right axis) is depicted in d. e Development of elevated CA15-3 concentrations in sera of four study patients before and after adoptive T cell transfer. Numbers below x axes depict days before (−) or after the treatment
Only three of all treated patients were HLA-A2 positive, among them two non responders and one responder (Table 1). While we did not detect MUC1-specific T cells in the blood of both non responders after treatment (Fig. 2c and data not shown), responder patient #09 showed a transient but considerable increase of MUC1-specific CD8 T cells 7 days after the transfer from 1.6 up to 3.6% of total CD8+ T cells (Fig. 2c, d), which corresponded to a simultaneous de novo detection of IFN-γ secreting MCF7-reactive T cells in the blood (Fig. 2d).
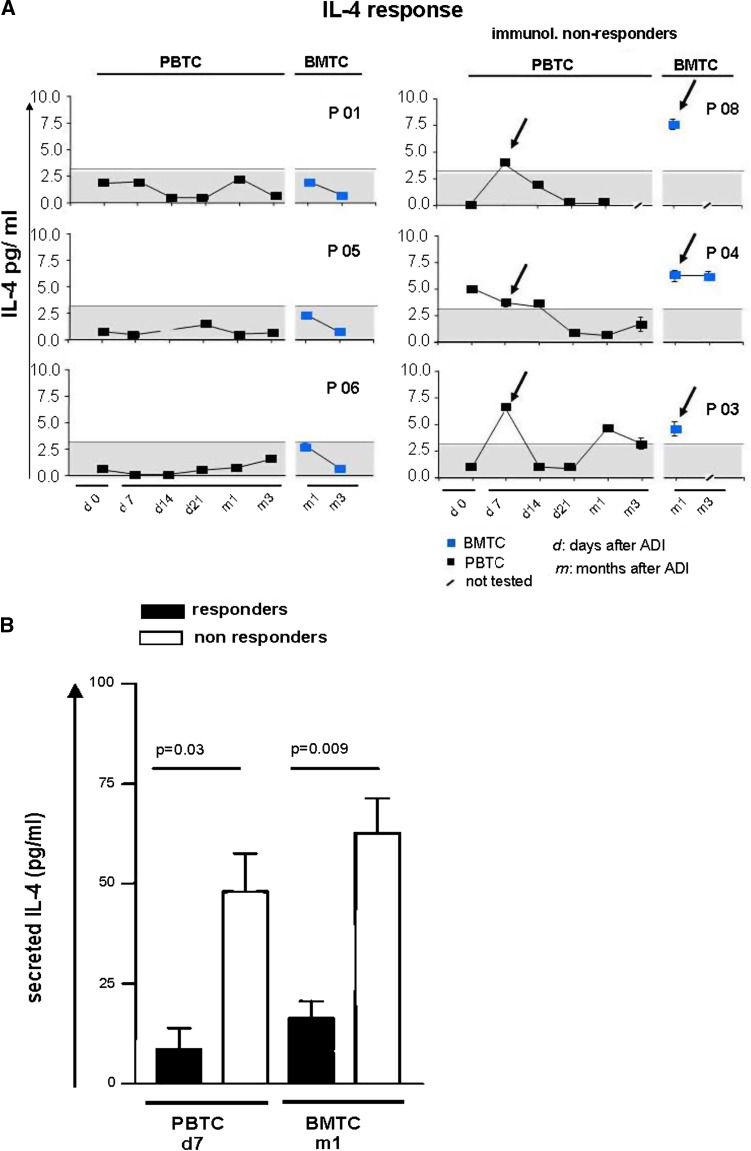
In addition to IFN-γ ELISPOT analyses we tested T cells from the BM and blood from six treated patients for MCF7-induced secretion of IL-4 into the ELISPOT culture supernatant by ELISA in order to assess a potential induction of tumour-reactive T2 type T cells (Fig. 3a, b). In all three tested ADI responders we did not detect an increase of tumour antigen-induced IL-4 secretion by blood-derived T cells above background levels at any time point after the treatment. In contrast, all three tested non responders showed increased tumour antigen-dependent IL-4 secretion particularly 7 days after treatment (P = 0.03). Interestingly, tumour antigen-induced IL-4 secretion was also consistently increased in BM T cells of non responders compared to responders (P = 0.01).
Fig. 3.
IL-4 responses. a, b Stimulated IL-4 secretion of bone marrow and blood T cells collected before and after cell transfer (ADI). IL-4 amounts were quantified by ELISA-assay from supernatants of 40 h stimulation cultures stimulation cultures containing tumour-lysate-pulsed autologous DC and T cells. a Individual results from three responders and three non responders. The maximum value detected in responder patients is depicted by an interrupted line and grey background. b Cumulative results of IL-4 contents in supernatants of bone marrow and blood T cells from responders (black bars) and non responders (white bars) at day 7 (PBTC) and 1 month (m1; BMTC) after T cell transfer. Significant differences between responders and non responders are depicted (Student’s T-test)
Taken together, half of the patients responded to the treatment with the appearance of circulating functional tumour-reactive type-1 T cells after 1 week, while non-responders showed simultaneously a dominant tumour antigen-induced T2 type T cell activity (P = 0.05, Fisher’s exact test).
Four of the 12 study patients, namely two responders and two non responders, showed enhanced serum concentrations of the tumour marker CA15-3 as a sign of disease activity before the treatment (Table 1). CA15-3 is part of the tumour antigen MUC1; therefore CA15-3 secreting tumour cells may be targets of tumour antigen-reactive CTL. We therefore followed the development of CA15-3 levels in these patients. Interestingly, we observed a treatment-associated CA15-3 decrease in responders but not in non responders (Fig. 2e).
Correlation of clinical immune responses with efficiency of therapeutic TC stimulation
In order to identify conditions that correlated with type-1 T-cell responses in the blood we compared pre-therapeutic clinical and laboratory parameters of the patients and their T cells, namely disease state, presence of bone metastases, elevation of serum tumour marker CA15-3, type of cytostatic pre-treatment, frequencies of tumour antigen-reactive BM T cells before treatment, amount of BM cells obtained from each patient, total numbers of stimulated T cells, numbers of transferred T cells, the T cell/DC ratio during therapeutic re-stimulation, estimated total numbers of tumour-reactive T cells in therapeutic stimulation cultures and expression of HLA-A0201 (Table 1).
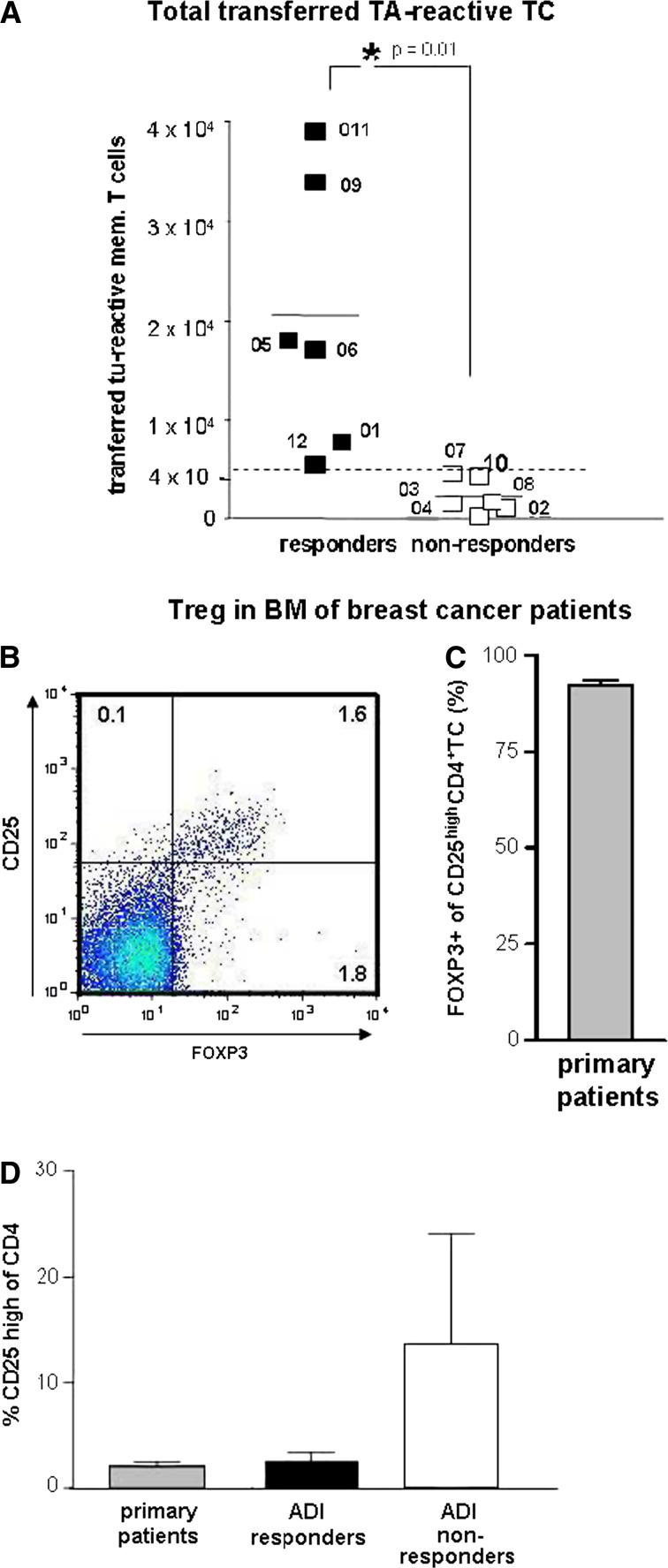
A positive correlation with type-1 T-cell responses in the blood was found for (1) the numbers of stimulated T cells; T cell stimulation cultures from five out of six responders contained more T cells than the median value of 2.25 × 107, while cultures from five out of six non responders contained less T cells (P = 0.04; Fisher’s exact test and P = 0.006; Student’s T-test), (2) for the numbers of transferred T cells (P = 0.04; Fisher’s exact test and not significant Student’s T-test) and (3) for the numbers of stimulated tumour-reactive BM T cells (as estimated by the product of tumour-reactive T cells/106 T cells × total T cells per stimulation culture (P = 0.001; Fisher’s exact test and P = 0.01; Student’s T-test; Table 1, Fig. 4a). Discrimination between responders and non-responders was best for the latter parameter, since all responders contained more tumour-reactive T cells in the stimulation cultures than the median value for the whole group (6.5 × 103) while all cultures of non-responders contained less than 6.5 × 103 tumour-reactive T cells. There was no correlation between expansion or reduction of T cells during the stimulation period and the immune response in the patients. The presence of bone metastases correlated negatively with type-1 T-cell responses in the blood (no responder but five out of six non-responders contained BM metastases; P = 0.01, Fisher’s exact test). Interestingly, the presence of bone metastases correlated also with a reduction of tumour-reactive T cells (P = 0.008; Fisher’s exact test) but not with reduced total numbers of stimulated T cells (P = 0.11; Fisher’s exact test) in the cultures according to respective median values (Table 1). None of the other parameters, including the disease state (stable disease versus progressive disease) or the frequencies of tumour-reactive BM T cells before treatment showed a significant correlation with the treatment outcome.
Fig. 4.
Pre-therapeutic immune parameters. a Estimated numbers of tumour-specific T cells transferred into patients (specific frequencies of tumour-reactive T cells in bone marrow before treatment × numbers of stimulated T cells). Black squares immunol responders, white squares immunol non-responders. Left scatter column immunol responders only, right column immunol non-responders only. P = 0.01, Student’s T-test. The numbers (Px) indicate different study patients. b–d Treg in bone marrow of breast cancer patients. Frequencies of Treg among CD4 T cells in bone marrow of primary breast cancer patients were determined by flowcytometry, using CD25 and FOXP3 as Treg marker (b). Since nearly all (92%) of CD25high CD4 T cells (N = 13) also expressed FOXP3 (c) Treg in bone marrow of study patients or primary breast cancer patients (d) were quantified by high expression of CD25. Bars represent mean ± SEM frequencies of Treg among CD4+ bone marrow T cells from primary (non metastasized) breast cancer patients (grey bar), ADI responders (black bar) and ADI non responders (white bar)
Taken together, the total numbers of stimulated and transferred tumour antigen-reactive BM T cells showed the closest correlation with detectable type-1 immune responses in the blood of the patients and appeared to be influenced by the presence of bone metastases.
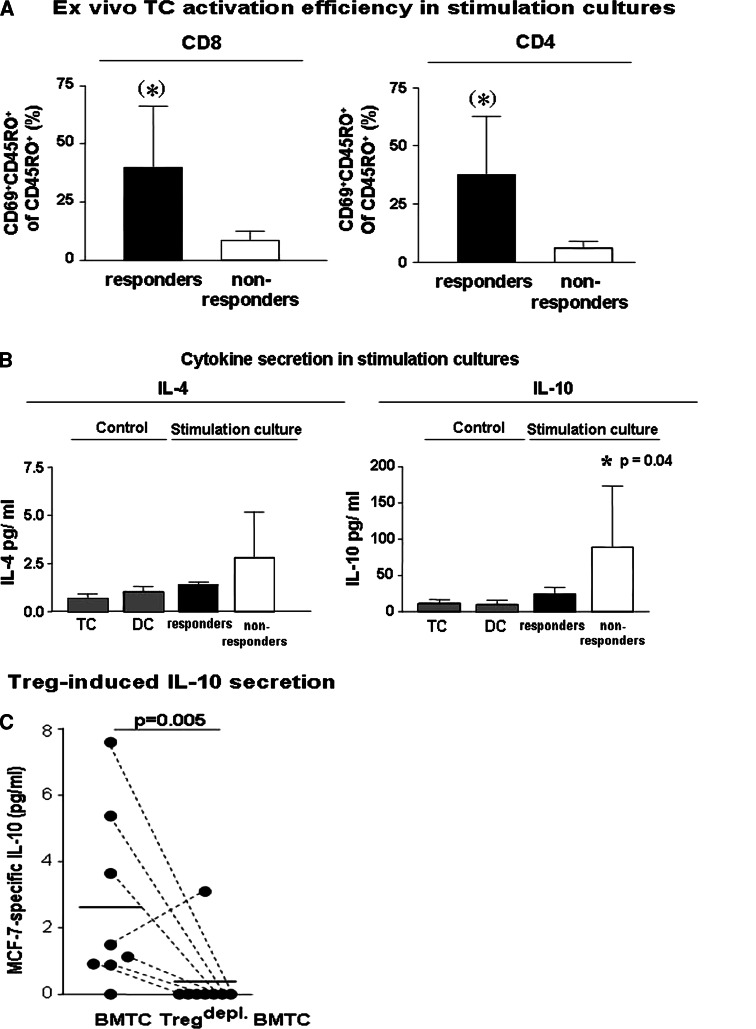
Tumours and their metastases attract Treg which possess the potential to effectively inhibit the activation and subsequent clonal expansion of T cells in vivo and in vitro [30]. We therefore analysed the proportions of Treg among BM T cells from primary breast cancer patients and from study patients and also the efficiency of T cell activation in the stimulation cultures using multicolour flowcytometry. Treg can be identified by flowcytometry through their expression of CD4, high levels of the IL-2 receptor alpha chain (CD25) and also by the expression of the transcription factor forkhead box P3 (FOXP3) (Fig. 4b). We first determined in BM of nine primary non metastasized breast cancer patients the frequencies of CD4+ CD25high FOXP3+ regulatory T cells by flowcytometry. BM Treg varied between 0.4 and 4.8% of T cells (mean 2.1 ± 0.4%). On average, 92 ± 3.5% of CD4+ CD25high T cells co-expressed FOXP3 (Fig. 4c). We now analysed the T cell stimulation cultures of altogether six study patients (three responders and three non responders) for the contents of Treg, using CD4 and CD25 high expression for detection of Treg. In these cultures, FOXP3 expression could not be analysed due to limitations of available fluorescence colours and limitations of cell numbers. The mean proportions of Treg among CD4 T cells in T cell cultures of non responders (13.9 ± 9.8%, all of them contained bone metastases) were approximately threefold higher compared to those of tested responders (2.8 ± 0.9%, none of them contained bone metastases) or of primary non metastasized breast cancer patients (Fig. 4d). Consistent with increased numbers of Treg, we observed a reduced efficiency of CD4 and CD8 memory T cell activation as quantified by expression of the early activation marker CD69 (Fig. 5a, P = 0.07 and 0.08, respectively).
Fig. 5.
a Ex vivo efficiency of memory T cell activation in therapeutic T cell stimulation cultures from responders (black bars) and non responders (white bars) as determined by mean + SEM proportions of early activated, CD69+ cells among CD45RO+ CD8TC or CD4 T cells. The asterisks depict a statistical trend towards increased CD69 expression by CD4 (P = 0.07) or CD8 (P = 0.08) T cell in stimulation cultures from responders. b Increased secretion of type-2 cytokines IL-4 (not significant) and IL-10 (P = 0.04, Student’s T-test) into the supernatants of 72 h therapeutic stimulation cultures of non responders (white bars) compared to responders (black bars) and corresponding unstimulated T cells or DC (control; grey bars). Graphs show mean + SEM values of cytokine concentrations as determined by ELISA. c MCF-7-dependent IL-10 secretion in short term (over night) stimulation cultures of bone marrow T cells from primary breast cancer patients containing autologous DC pulsed with MCF7 lysate or negative control antigen U937 lysate together with isolated total bone marrow T cells (BMTC) or BMTC depleted from contaminating Treg by anti CD25-coated magnetic beads (Tregdepl. BMTC) as calculated by subtraction of IL-10 concentrations in U937-stimulated cultures (background) from IL-10 levels in MCF-7 stimulated T cell cultures (P = 0.005, paired Student’s T-test). Dots represent the values from independent experiments with different breast cancer patients (n = 8). Negative results are depicted at the bottom line. Corresponding dots are connected by interrupted lines
Upon activation, Treg populations can secrete high levels of the immune suppressive cytokine IL-10 which can inhibit the activation of type 1 but not type-2 T cells. In accordance to the increased numbers of Treg in T cell cultures of non responders, we also observed a significantly increased release of IL-10 (P = 0.04) and also increased IL-4 secretion (not significant) into the supernatants of respective 72 h T cell stimulation cultures (Fig. 5b). In order to evaluate the influence of BM Treg on the IL-10 secretion during TC stimulation, we depleted Treg from BM T cells of primary breast cancer patients using anti CD25 mAb labelled magnetic beads and stimulated the T cells during short term (over night) cultures with MCF-7 pulsed autologous DC. Under these conditions, the depletion of Treg abrogated MCF-7-dependent IL-10 secretion (Fig. 5c, P = 0.005).
Discussion
In this small pilot study we evaluated the feasibility of a short term ex vivo reactivation of pre-existing tumour-reactive memory T cells in patients with late stage metastasized breast cancer. All 12 patients that were included into the study could be treated according to the study protocol without signs of treatment related toxicity, while 5 patients who met the inclusion criteria could not be included due to death or poor clinical status. Fifty percent of the patients responded to the treatment with the transient presence of type-1 MCF7-reactive T cells in the blood. Due to the lack of suitable full length proteins of breast cancer associated antigens at the time of the design of our study, we used MCF7-lysate as test antigen for the immune monitoring after the treatment. Therefore, our data do not formally proof that the procedure is suitable to reactivate BM memory T cells with specificity for breast cancer antigens. In order to address this issue, we quantified in HLA-A2 positive patients MUC1-specific CD8 T cells before and after the treatment by tetramer analyses. Although only one responder patient was HLA-A2 positive, we could demonstrate in the blood of this patient a considerable increase of MUC1-specific CD8 TC 7 days after the treatment—concomitant with an increase in MCF7-reactive T cells as quantified by IFN-γ ELISPOT assay. Therefore, T cell reactivation with an allogeneic tumour cell line might be suitable to activate TA-specific T cells in case that complex preparations of more defined tumour antigens or tumour cell mRNA are not accessible for T cell stimulation in the future.
Regarding the immunological effects of the treatment we found an altogether consistent pattern of either dominant type-1 or type-2 tumour-responses which were determined already during therapeutic T cell stimulation: Occurrence of type-1 tumour-reactive T cells in the blood of treated patients was associated with an efficient therapeutic T cell activation ex vivo and always found after transfer of an estimated dose of more than 6.5 × 103 tumour-reactive T cells. In contrast, those patients who did not respond with type-1 T-cell responses in the blood showed tumour antigen-dependent type-2 responses instead as indicated by increased IL-4 secretion of tumour antigen-stimulated T cells in the blood and BM after the transfer. These patients differed from responders by the presence of bone metastases, increased numbers of Treg and increased amounts of IL4 and IL-10 in the stimulation cultures. All of them received less than 6.5 × 103 tumour-reactive type-1 T cells.
Total amounts of reactivated type-1 T cells depend on activation efficiency and T cell proliferation. Both features can be impaired in the presence of Treg [31]. Indeed, when we depleted Treg from BM T cells before their stimulation with tumour antigens, we observed a significant reduction of tumour antigen-dependent IL-10 secretion. Together with TGFβ1, IL-10 is one of the major effector cytokines of Treg [31]. IL-10 inhibits in vitro DC maturation, efficient adaptive T-cell responses and the secretion of IFN-γ by effector TC [32, 33], while it has no effect on the secretion of the type-2 cytokine IL-4. Elevated plasma levels of IL-10 correlated with poor prognosis which was ascribed to increased tumour loads in metastasized patients [34]. In contrast to IL-10, dominant IFN-γ secretion, as observed in responder patients, inhibits the activation of TH2 cells. This may explain the lack of IL-4 induction in BM and blood T cell activation cultures of responder patients before and after the treatment. Taken together, our data suggest a dominant activation of Treg in some cultures. This might have been facilitated by the use of immature DC and could be prevented in the future by addition of further DC maturation stimuli. In addition, patients might benefit from ex vivo Treg depletion prior to T cell stimulation in future adoptive T cell transfer studies.
In the blood we detected tumour-reactive T cells up to 7 days after the transfer.
Their observed frequencies of 100–510/106 T cells in the blood are remarkable since approximately 1 × 104 applied specific T cells, diluted in an approximated blood volume of 6,000 ml would be virtually undetectable by ELISPOT assay. This suggests a phase of T cell expansion, presumably in lymphatic organs. Upon reactivation, memory T cells undergo a 3–5 day period of rapid expansion in lymphoid organs which is followed by the acquisition of effector function, emigration into the blood, tumour infiltration and tumour rejection [16, 20–22]. It appears therefore possible that in our study circulating tumour-reactive T cells might have re-entered lymphatic organs or immigrated into peripheral sites, as also suggested by the observation of CA15-3 reduction in two patients. In xenotransplant models of breast cancer [15, 16] and in murine melanoma [21] tumour infiltration and -rejection by transferred tumour-reactive memory T cells was similarly observed after 7–10 days. Clinical studies with excessively expanded tumour antigen-specific T cell lines demonstrated that transferred T cells were detectable in the circulation for only a short period of several days [2, 35] but strongly infiltrated the liver, spleen and the BM where they mediated rejection of disseminated breast tumour cells, while established liver metastases were only scarcely infiltrated [35]. The targeting of activated memory T cells into solid tumour tissue thus appears to be a major challenge of future immunotherapeutic approaches.
A major caveat of our approach was the overall low number of tumour-reactive T cells obtained by BM aspiration. Besides the major technical restrictions of BM aspiration this can be most likely be attributed to the late stage situation of the patients after multiple palliative cytostatic treatments since we observed much higher frequencies of tumour-reactive T cells in primary metastasized breast cancer patients. Insufficient numbers of tumour-reactive T cells were particularly obtained from patients with bone metastases and may be caused by increased frequencies of Treg in the BM of these patients. Therefore, potential future approaches need to address the problem of collecting sufficient amounts of tumour-reactive T cells for the treatment. We observed in several patients that tumour-reactive T cells were mobilized from the BM into the blood by leukapheresis. The latter procedure provides nearly unlimited numbers of T cells and appears a promising source of tumour antigen-reactive T cells in the future.
We did not observe significant changes in the frequencies of tumour-reactive T cells in the BM one and three months after the treatment. Thus, the BM repertoire of tumour antigen-reactive T cells appears to be mainly uninfluenced by the treatment and could be further exploited for serial T cell transfers. This may be of importance since a single T cell transfer will presumably not cause a long-lasting fundamental change of the patients’ immunological or clinical situation.
Conclusions
We conclude that the BM repertoire of tumour-reactive memory T cells of late stage metastasized breast cancer patients can be a T cell source for ADI. The immunological effectiveness appears to depend on the disease state of the patients and on the number of transferred T cells. Tumour antigen-reactive BM memory T cells appear to be capable of rapid proliferation and immigration into the blood circulation as a prerequisite for tumour immune rejection. Future improvements might include a selection of defined tumour antigens together with adjuvants preventing the re-activation of T2 type responses during stimulation, the application of higher cell numbers or the development of in situ techniques for mobilization of BM resident tumour-reactive memory T cells as well as the depletion of Treg from BM preparations before their ex vivo activation.
Footnotes
Florian Schuetz and Katrin Ehlert contributed equally to the study.
References
- 1.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh LL, Bonasio R, Mazo IB, Halin C, Cheng G, van der Velden AWM, Cariappa A, Chase C, Russell P, Starnbach MN. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nat Immunol. 2005;6:1029–1037. doi: 10.1038/ni1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hammerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 5.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Sipkins DA, Wie X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khazaie K, Prifti S, Beckhove P, Griesbach A, Russell S, Collins M, Schirrmacher V. Persistence of dormant tumor cells in the bone marrow of tumor cell-vaccinated mice correlates with long-term immunological protection. Proc Natl Acad Sci USA. 1994;91:7430–7434. doi: 10.1073/pnas.91.16.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller M, Gounari F, Prifti S, Hacker HJ, Schirrmacher V, Khazaie K. EblacZ tumor dormancy in bone marrow and lymph nodes: active control of proliferating tumor cells by CD8+ immune T cells. Cancer Res. 1998;58:5439–5446. [PubMed] [Google Scholar]
- 9.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heth WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 10.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 11.Schoenberger SP, Toes RE, Van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 12.Kammertoens T, Schuler T, Blankenstein T. Immunotherapy: target the stroma to hit the tumor. Trends Mol Med. 2005;11:225–231. doi: 10.1016/j.molmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa H, Kato T, Tawara I, Ikeda H, Kuribayashi K, Allen PM, Schreiber RD, Old LJ, Shiku H. IFN-gamma controls the generation/activation of CD4+ CD25+ regulatory T cells in antitumor immune response. J Immunol. 2005;175:4433–4440. doi: 10.4049/jimmunol.175.7.4433. [DOI] [PubMed] [Google Scholar]
- 14.Schirrmacher V, Feuerer M, Fournier P, Ahlert T, Umansky V, Beckhove P. T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 2003;9:526–534. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 16.Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92–98. doi: 10.1016/S0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 19.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005;175:1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dailey M. Expression of T lymphocyte adhesion molecules: regulation during antigen-induced T cell activation and differentiation. Crit Rev Immunol. 1998;18:153–184. doi: 10.1615/critrevimmunol.v18.i3.10. [DOI] [PubMed] [Google Scholar]
- 24.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 25.Bai L, Beckhove P, Feuerer M, Umansky V, Choi C, Solomayer FS, Diel IJ, Schirrmacher V. Cognate interactions between memory T cells and tumor antigen-presenting dendritic cells from bone marrow of breast cancer patients: bidirectional cell stimulation, survival and antitumor activity in vivo. Int J Cancer. 2002;103:10–20. doi: 10.1002/ijc.10781. [DOI] [PubMed] [Google Scholar]
- 26.Sommerfeldt N, Schütz F, Sohn C, Förster J, Schirrmacher V, Beckhove P. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res. 2006;66:8258–8265. doi: 10.1158/0008-5472.CAN-05-4201. [DOI] [PubMed] [Google Scholar]
- 27.Kammerer U, Thanner F, Kapp M, Dietl J, Sutterlin M. Expression of tumor markers on breast and ovarian cancer cell lines. Anticancer Res. 2003;23:1051–1055. [PubMed] [Google Scholar]
- 28.Jiang XP, Yang DC, Elliott RL, Head JF. Vaccination with a mixed vaccine of autogenous and allogeneic breast cancer cells and tumor associated antigens CA15–3, CEA and CA125-results in immune and clinical responses in breast cancer patients. Cancer Biother Radiopharm. 2000;15:495–505. doi: 10.1089/cbr.2000.15.495. [DOI] [PubMed] [Google Scholar]
- 29.Sommerfeldt N, Beckhove P, Ge Y, Schütz F, Choi C, Bucur M, Domschke C, Sohn C, Schneeweis A, Rom J, Pollmann D, Leucht D, Vlodavsky I, Schirrmacher V. Heparanase: a new metastasis-associated antigen recognized in breast cancer patients by spontaneously induced memory T lymphocytes. Cancer Res. 2006;66:7716–7723. doi: 10.1158/0008-5472.CAN-05-2363. [DOI] [PubMed] [Google Scholar]
- 30.Nummer D, Suri-Payer E, Schmitz-Winnenthal H, Bonertz A, Galindo L, Antolovich D, Koch M, Büchler M, Weitz J, Schirrmacher V, Beckhove P. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J Natl Cancer Inst. 2007;99:1188–1199. doi: 10.1093/jnci/djm064. [DOI] [PubMed] [Google Scholar]
- 31.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+ CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 32.Moore KW, de Waal MR, Coffmann RL, O’Gara A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 33.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24:36–43. doi: 10.1016/S1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 34.Mocellin S, Ohnmacht GA, Wang E, Marincola FM. Kinetics of cytokine expression in melanoma metastases classifies immune responsiveness. Int J Cancer. 2001;93:236–242. doi: 10.1002/ijc.1328. [DOI] [PubMed] [Google Scholar]
- 35.Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann H, Nährig J, Fend F, Weber W, Busch D, Peschel C. Adoptive transfer of autologous, HER2-speciWc, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]