Abstract
Treatment of metastatic cancer mainly relies on chemotherapy. Chemotherapeutic agents kill tumor cells by direct cytotoxicity, thus leading to tumor regression. However, emerging data focus on another side of cancer chemotherapy: its antitumor immunity effect. Although cancer chemotherapy was usually considered as immunosuppressive, some chemotherapeutic agents have recently been shown to activate an anticancer immune response, which is involved in the curative effect of these treatments. Cancer development often leads to the occurrence of an immune tolerance that prevents cancer rejection by the immune system and hinders efficacy of immunotherapy. Cancer cells induce proliferation and local accumulation of immunosuppressive cells such as regulatory T cells and immature myeloid cells, and prevent the maturation of dendritic cells and their capacity to present tumor antigens to T lymphocytes. Many anticancer cytotoxic agents interfere with the molecular and cellular mechanisms leading to tumor-induced tolerance. They can restore an efficient immune response that contributes to the therapeutic effects of chemotherapy. These findings open a novel field of investigations for future clinical trial design, taking into account the immunostimulatory capacity of chemotherapeutic agents, and using them in combined chemo-immunotherapy strategies when tumor-induced tolerance is overcome.
Keywords: Apoptosis, Cell death, Danger signals, Chemotherapy, Senescence
Introduction
Clinical applications of tumor immunology in the field of cancer chemotherapy are presently limited. The complex molecular mechanisms involved in cell transformation and cancer lead to the development of neoantigens and danger signals on cancer cells, which should give rise to immune rejection of the tumor (immunosurveillance). However, cancer cells may escape this rejection by limiting tumor antigen expression (immunoediting) and mainly by inducing active immune tolerance mechanisms. These mechanisms include the proliferation and local accumulation of immunosuppressive tolerogenic cells, including regulatory T cells and immature myeloid cells. This tolerance prevents cancer rejection by the immune system and blunts efficiency of immunotherapy.
Treatment of metastatic solid cancers essentially relies on cytotoxic drugs that kill tumor cells or hinder their proliferation. Considering their toxicity on hematopoietic cells, chemotherapeutic agents are often considered as immunosuppression inducers (reviewed in [1]). Indeed, in vitro, many drugs used for cancer therapy exhibit direct cytotoxic effects on cells of the immune system such as T lymphocytes [2, 3]. Moreover, in humans, many cytotoxic drugs may induce a immunosuppression state: for example, temozolomide [4] or fludarabine [5] may induce lymphopenia, causing a T lymphocyte-related immunosuppression state. Cyclophosphamide and methotrexate are also used as immune suppressors in the treatment of autoimmune diseases [6, 7] with, however, specific administration schedules.
In contrast, some chemotherapeutic agents display some positive immunological effect. On the one hand, chemotherapy could deplete immunosuppressive cells such as CD4+ CD25+ regulatory T cells (Treg) and Myeloid-Derived Suppressor Cells (MDSC) and could enhance latent antitumor immune response or synergize with a tumor vaccine [8–10]. On the other hand, chemotherapy-induced cell death may release tumor antigens that could be uptaken by antigen presenting cells (APC), processed and presented to naive T cells. Therefore, the signals released by killed tumor cells would impact on phagocytosis and/or antigen processing, or maturation and trafficking of dendritic cells.
This review will describe the ability of some chemotherapeutic cytotoxic agents to eliminate immunosuppressive cells, and the molecular mechanisms involved in this immunogenic response.
Modulation of tolerance pathways
Low dosage of cyclophosphamide, as an efficient chemotherapeutic regimen to blunt CD4+ CD25+ regulatory T cells?
The DNA alkylating molecule, cyclophosphamide (CPM), has been used for decades as part of chemotherapeutic regimens in numerous malignancies. The immunomodulatory effects of CPM have been discovered 26 years ago by Robert North’s team [11]. In mouse models, they described a “suppressor CD4+ T cells” population, which inhibited T cell-mediated immunity against tumors, and that could be removed by administration of low dose of CPM [11, 12]. However, the phenotype expressed by these suppressor cells was CD4+ T cells mainly containing nonsuppressor cells. “T suppressor cells” were subsequently characterized by Sakaguchi’s laboratory [13] and renamed “CD4+ CD25+ regulatory T cells” (Treg). Treg are defined as a subpopulation of suppressor T cells that mediate immune tolerance by inhibiting virtually any immune cells [14, 15]. They represent 1–3% of circulating CD4+ T cells in humans and about 10% of splenic CD4+ T cells in rodents. Mouse and human Treg were found to express Foxp3, a specific forkhead transcription factor that controls both their development and function [16]. Treg play key roles in many physiopathological settings including cancer. During malignancy development, Treg accumulate in the blood, draining lymph nodes (LN) and tumors in humans [17, 18] and rodents [8, 19–21]. In patients with ovarian, breast, pancreatic carcinoma or glioblastoma, infiltration of tumor by Treg correlates with a poor prognosis [17, 18, 22, 23].
North’s pioneering studies in the 1980s suggested that suppressive T cell function may be electively inhibited in tumor hosts receiving low-dose CPM treatment [11, 24]. To confirm these results in the updated light of Treg biology, we design a strategy for studying the impact of low dosage of CPM in rodent tumor models. In inbred rats bearing an established subcutaneous tumor obtained from a syngeneic colon carcinoma, a single injection of low-dose CPM (25 mg/kg of body weight) decreased selectively the percentage of CD25+ Treg among the CD4+ lymphocytes on the seventh day following treatment. This allowed a whole tumor cell vaccine immunotherapy to completely eradicate established tumor in all treated animals [8], whereas the tumor vaccine or the CPM alone were unable to cure any. These results were confirmed in several mouse tumor models [25–29] where CPM decreased the Treg number, restored effector T cells functions and even restored efficacy of dendritic cells vaccine [30]. Several hypotheses may explain the high sensitivity of Treg to CPM. First, in tumor-bearing hosts, tumor drives permanently the proliferation of Treg, a status that could contribute to their selective sensitivity to CPM [31, 32]. Second, the sensitivity of Treg to CPM could be linked to their selective Foxp3 expression, which is associated with higher expression of proapoptotic molecules [33], which sensitize Treg to apoptosis. Additionally, forced expression of Foxp3 in a transfected leukemic T cell line increased their sensitivity to CPM (Ghiringhelli, personal communication).
Low dosing of CPM not only decreased the number, but also inhibited the suppressive function of the residual Treg [27, 34]. Treg capacity to inhibit CD8 or CD4 T cells functions was markedly decreased when Treg were obtained from mice treated with CPM compared to those obtained from naïve mice [34]. Finally, the in vivo relevance of CPM-induced Treg depletion was studied by Taïeb et al. [34], who demonstrated that one injection of low-dose CPM (100 mg/kg of body weight in mouse) synergized with an immunotherapeutic regimen consisting of tumor peptide-loaded exosomes, combined with CpG ODN, resulted in the regression of established tumors.
In humans, low dosages of CPM (300 mg/m2) administrated in cancer patients 3–4 days prior to antigen exposure promoted immune response rather than tolerance as demonstrated by their capacity to potentiate hypersensitivity responses assays [35]. The feasibility of combining low dose of CPM and tumor vaccination has been attempted in clinical trials, but such studies lacked statistical power [36–40]. Nevertheless, in a study of 42 metastatic breast cancer patients receiving a vaccine composed of a synthetic sialyl-Tn epitope linked to Keyhole Limpet Hemocyanin (KLH) and an adjuvant, an intravenous injection of CPM given prior to vaccination significantly increased median survival from 12 to 20 months [41]. In these studies, the biological effects of CPM on effector and regulatory T cells were not analyzed.
Administration of low dose of CPM at regular intervals as a “metronomic regimen” in patients bearing metastatic breast, prostate or ovarian carcinoma demonstrated a clinical efficacy [42–44]. This effect was attributed to some antiangiogenic properties of metronomic regimen [44]. Taking into account the immunological effect of CPM on Treg in rodent tumor models, we performed a phenotypical and functional immunomonitoring in ten patients with advanced metastatic cancers receiving an oral CPM treatment on a metronomic schedule. The number of peripheral blood Treg decreased dramatically after 1 month of treatment [9]. Moreover, peripheral T cells proliferative capacity and NK cell cytotoxicity were enhanced. Furthermore, two variants of treatment were tested: some patients received CPM at 100 mg/day 1 week of 2, while other patients received twice the CPM dose, that is, 100 mg/twice a day, 1 week of 2. The precise dosing of CPM was crucial to selectively deplete Treg, while sparing effector T cells, and the specificity of Treg depletion was lost at a higher CPM dose. At a high CPM dose, patients exhibited significant decrease in whole lymphocytes, CD4+ T cells and CD8+ T cells counts. Our results underline that CPM given at optimal dosing in cancer patients induced a specific depletion of Treg. Moreover, lowering the CPM regimen to 50 mg/day was equivalent to 100 mg/day 1 week of 2 (C Ménard, unpublished result). The antiangiogenic effect of CPM given as a metronomic regimen [44] could potentiate its enhancing effect on the tumor immune response as suggested by combined therapy associating an inhibitor of angiogenic receptor and an immunostimulator protein [45].
Could metronomic CPM regimen improve the effect of immunotherapy ?
In mice bearing B16F10 melanoma, CPM given daily at a low dosage according to a metronomic regimen enhanced the effect of an immunization protocol consisting of injection of tumor recombinant DNA followed by recombinant modified vaccinia virus Ankara strain [46]. Combining tumor vaccine with metronomic CPM regimen dramatically improved the antitumoral activity compared to treatments administered alone, or to a combinaison of tumor vaccine with a bolus dose of CPM. In humans, we previously demonstrated that human Treg can use their membranous TGF-β for blunting NK cell proliferation, cytotoxicity and IFN-γ secretion [32]. In patients treated with CPM metronomic regimen, Treg depletion should allow NK cell-based immunotherapy to be more efficient by a phenomenom known as “releasing the brake”. Borg et al. previously described that imatinib mesylate (IM), a tyrosine kinase inhibitor, in association with its direct antitumor effect could also stimulate NK functions in Gastro-Intestinal Stromal Tumor (GIST)-bearing patients [47]. Consequently, a treatment associating IM with metronomic CPM was attempted in relapsing GIST patients, whose tumor became resistant to the direct cytotoxic effect of IM. These patients had been treated with IM for more than 1 year and exhibited tumor progression under several lines of treatment (IM 400 mg, then 800 mg daily and ultimately sunitinib). IM was re-administered at 400 mg daily in association with a low dose of CPM. Of seven treated patients, disease stabilization was achieved for several months in two of them, and partial response following RECIST criteria was obtained in two other patients (C Ménard, unpublished data). These data strengthen the proof of concept that a metronomic regimen of CPM may be associated with immunotherapy and may enhance its efficacy.
Elimination of Myeloid-Derived Suppressor Cells
Myeloid-Derived Suppressor Cells (MDSC) represent a population of immature myeloid cells including the precursors of macrophage, granulocyte and dendritic cells, which might suppress T-dependent immune response. In mice, key phenotypic markers are CD11b and Ly6G/C. MDSC accumulate in the lymphoid organs of tumor-bearing mice, thus contributing to tumor-induced immunosubversion [48, 49]. These cells can directly control T cell response, but induce also the proliferation of Treg through TGFβ production [50]. Thus, a cytotoxic drug that electively eliminates MDSC while preserving T cells subset may enhance latent tumor immunity and tumor vaccine efficacy. Gemcitabine, a synthetic pyrimidine nucleoside analog, commonly used for the treatment of pancreatic, breast and lung carcinoma, was tested with this aim. Gemcitabine, at 120 mg/kg body weight, specifically reduced the number of MDSC found in the spleen of BALB/c and C57Bl/6 mice bearing large tumors, but did not reduce CD4 and CD8 T cells, NK cells, macrophage and B cells [10, 51, 52]. Interestingly, gemcitabine used in association enhanced the treatment efficacy of antitumor vaccine, or of intratumoral IFN-β gene therapy [52] or dendritic cells vaccine [10].
Recently, amino-biphosphonates, commonly used for treatment of bone metastases, were found to decrease the total number of MDSC and to inhibit matrix-metalloproteinase 9 (MMP9) expression on tumor cells and on stromal cells. Considering that MMP9 controls availability of vascular endothelial growth factor (VEGF) to its receptor [53], and that VEGF is necessary for MDSC expansion [54], amino-biphosphonates are involved in MDSC inhibition.
Molecular mechanisms of immunogenicity of cytotoxic drugs
The problem of tumor cell death
Interaction between tumor cell death and immune system is complex. While apoptotic cell death of normal tissue must be ignored to avoid the development of autoimmune diseases, apoptosis of virally infected cells has to be considered. In the setting of solid tumor, dealing with the immunogenicity of tumor cells death induced by chemotherapy seems surprising. Solid tumor growth is generally associated with the death of many tumor cells, mostly in the central area of the tumor because of a lack of vascularization. Lots of necrotic and apoptotic cells are found in these tumors. So, it is difficult to think that the immunological effect of chemotherapy is only related to its ability to trigger cell death and enhance the antigen delivery.
However, the type of cell death induced by chemotherapy in vivo may be a determinant factor for triggering immunogenicity of cell death. High concentrations of various cytotoxic agents induce apoptotic cell death of tumors. But, at pharmacological relevant concentrations, these agents rather inhibit tumor cell proliferation and induce morphological and molecular changes characteristic of senescence or mitotic catastrophes [55–58]. For example, P53-mediated senescence induces the release of proinflammatory molecules by tumor cells and further the involvment of the immune system. These cytokines recruit NK cells, neutrophils and macrophages, thus leading to tumor eradication and modulate in a large part T-dependent immune response [59]. DNA damaging agents used for cancer chemotherapy such as topoisomerase inhibitors and platinum derivates stimulate the production of tumor suppressor proteins such as ATM (Ataxia Telangiectasia Mutated) and p53. The DNA damage triggers tumor cells to express ligand for NKG2D, an activating receptor of NK, NKT, γδT cell components of the innate immune system that could trigger a Th1 immune response [60]. Altogether, these data argue for a potential link between chemotherapeutic induced cellular senescence and inflammatory response.
Sensitization of tumor cells to immune effectors through modulation of death receptor expression by chemotherapy
The type I transmembrane receptor Fas and DR4/DR5 are molecules belonging to the tumor necrosis factor/nerve growth factor receptor family. These receptors are important mediators of immunologically mediated cell apoptosis. Their respective ligands, Fas-L and TRAIL, are expressed by activated CD8+ T and NK lymphocytes. Subtoxic concentrations of chemotherapeutic drugs could restore the response to Fas-L and TRAIL in cancer cells, which are not spontaneously sensitive to these cytokines [61, 62]. Thus, chemotherapeutic drugs such as cisplatin, doxorubicin, mitomycin C, fluorouracil, and camptothecin, which exert direct cytotoxic effects, also exhibit an indirect cytotoxic activity by “preparing” tumor cells to their own elimination by immune cells such as NK or cytotoxic T lymphocytes using a Fas or TRAIL-dependent pathway.
Nonspecific activation of macrophage innate immune effector by chemotherapeutic agents
Many reports described that intraperitoneal (i.p.) injection of chemotherapeutic agents such as anthracycline (doxorubicin) or mitomycin C could enhance the capacity of peritoneal macrophages to kill tumor cells [63–67], at least in rodents. Peritoneal macrophage cytotoxicity was dramatically enhanced in different tumor cell lines, 1–4 days after i.p. injection. In contrast, cyclophosphamide, vincristine, and methotrexate did not enhance macrophage cytotoxicity [66], but increased the production of inflammatory cytokines such as IL-1, TNF-α and IFN-γ [67, 68]. The mechanisms of macrophage activation remain unclear. Previous data from our group displayed that peritoneal macrophages collected 24 h after an i.p. injection of doxorubicin were cytotoxic against tumor cells, whereas in vitro treated macrophages never exhibited tumoricidal activity. Macrophages incubated with doxorubicin in vitro accumulated the drug in their nucleus, whereas macrophages from animals receiving doxorubicin in vivo accumulated the drug in cytoplasmic vacuoles, which were transferred to contiguous cancer cells and induced their death. Further analyses demonstrated that doxorubicin first concentrated in mast cell granules and was then released and captured through pinocytosis by peritoneal macrophages [64].
Presently, investigations need to assess whether similar mechanisms involving tumor-associated macrophages, and perhaps undifferentiated myeloid cells infiltrating the tumors, are involved in the efficacy of chemotherapy using anthracyclines or mitomycin-C.
Chemotherapy can also enhance the immune response against cancer by modifying the interaction between tumor cells and dendritic cells (DC), which play a key role in antigen presentation to T lymphocytes. In addition, chemotherapy can enhance endocytosis of tumor cells by dendritic cells. Finally, tumor cells are involved in the production of “danger signals” that are required for dendritic cell maturation and tumor antigen presentation to T cells [69].
Some “eat-me” signal inducing phagocytosis by dendritic cells
To trigger Th1 immunity, dead tumor cells or their fragments (apoptotic bodies) should preferentially undergo phagocytosis by dendritic cells rather than by macrophages or neutrophils that would rapidly degrade tumor antigens, preventing their capacity to prime T cells [70]. Conventional apoptosis leads to exposure of phosphatidylserine at the surface of dying tumor cells, which facilitates the uptake by macrophages rather than dendritic cells, and enhances the production of immunosuppressive factors such as TGF-β and IL-10 [71]. Moreover, binding to phosphatidylserine stimulates dendritic cells to secrete MFG-E8, an opsonin that promotes phagocytosis of apoptotic bodies by dendritic cells and macrophages, but mediates tolerance by inducing regulatory T cells that inhibit Th1 immune response [72].
On the contrary, other plasma membrane signals may trigger dying tumor cells to activate dendritic cells induction of polarized Th1 immune response. Calreticulin, a calcium-binding protein promotes this mechanism in mice tumor models. Anthracyclines and platinum derivatives translocate calreticulin from the cytoplasm to the plasma membrane of dying cells. When combined to a decrease of CD47 at the cell surface, calreticulin translocation facilitates the phagocytosis of dying cells by dendritic cells and macrophages [73, 74]. Moreover, calreticulin exposure on the dying cell surface is associated with the development of a Th1 immune response, suggesting that calreticulin dependent phagocytosis of dying cells in an important event for the induction of antitumor immune response. Nevertheless, all experiments assessing the effect of calreticulin on phagocytosis have limitations, as they used DC obtained from naïve animals instead of tolerized dendritic cells isolated from established tumors. Furthermore, the contribution of calreticulin to chemotherapy immunogenicity in human tumors remains to be established.
An antigen processing signal: HMGB1
Induction of an efficient T cell-dependent immune response requires first that immature dendritic cells collect nominal antigens, and secondly that they differentiate in mature dendritic cells capable of activating Th1 cells. Agents inducing this maturation are frequently exogenous danger signals that activate receptors at the surface of dendritic cells, such as components of virus and bacteria signaling through Toll-like receptors [75]. Recently, Toll-like receptors (TLR) were also involved in the recognition of endogenous danger signal provided by dying cells [69]. Chemotherapy immunogenicity may be related to the ability of tumor cell death to release danger signals that could in turn contribute to the development of anticancer immunity [76]. Our previous studies demonstrated that the TLR-4 receptor was involved in the T cell dependent immunity induced by oxaliplatin and doxorubicin [77, 78]. In several murine tumor models, the therapeutic effect of cytotoxic agents was drastically hampered in TLR-4-deficient mice compared with wild-type mice, thus underlining the requirement of TLR-4 expression to control chemotherapy efficacy. Interestingly, TLR-4 expression is only required in DC. TLR-4-deficient DC were not impeded in their maturation but only in their capacities to effectively present and cross-present tumor antigen to CD4 and CD8 T cells, respectively. At the molecular level, TLR-4 signaling delays fusion between phagosome and lysosome in dendritic cells, thus preventing antigen degradation and increasing the number of MHC-tumor antigen peptide complexes expressed on the cell surface of DC [78, 79]. The TLR-4 ligand released by tumor cells injured by chemotherapy was identified as the high mobility group box 1 (HMGB1) protein, a recently identified alarmin. However, since HMGB1 per se does not promote full-blown DC maturation, signals delivered by dying tumor cells that engage DC maturation and Th1 polarization remain to be established. In clinic, analyses of a serie of breast cancer patients revealed that a loss of function polymorphism in the TLR-4 gene was an independent factor of poor prognosis in response to chemotherapy [78]. Thus, clinically used anticancer drugs also seem to mediate their cytotoxic effects through the acquired immune system [80].
Maturation of DC Th1 polarization
In some cases, chemotherapeutic agents could directly activate the innate immune system. For instance, paclitaxel binds to mouse TLR4, but not to human TLR4, and so can mimic bacterial LPS by activating mouse macrophages and DCs in a MyD88 dependant pathway [81]. Moreover, some vascular disrupting agents such as DMXAA (5,6-dimethylxanthenone-4-acetic acid) could display positive immunological effects: in tumor models, DMXAA promote tumor bed infiltration by CTL and myeloid cells, probably by activation of dendritic cells through a Myd88 independent pathway [82, 83]. In opposite cases, only factors released by tumor cells are involved in the activation of the innate immune system. Deposit of uric acid crystals by dying cells was recently reported to be a danger signal that activates DC maturation [84]. Depletion of uric acid using allopurinol decreases CD8+ T cells response and inhibits tumor regression. Tumor regression is associated with high tumor levels of uric acid, and intratumoral injections of uric acid accelerates tumor regression [85]. As uric acid could be released by tumor cells after chemotherapeutic injuries, we hypothesized that the immune response might only be elicited when uric acid blood level reaches its crystallization point. In DC, uric acid engages the caspase-1 activating NALP3 inflammasome, a complex of proteins aimed at activating caspase-1, thus leading to the cleavage of pro-IL-1 and pro-IL-18 to bioactive IL-1 and IL-18. Any deficiency in one protein involved in the inflammasome in macrophages blunts their capacity to produce IL-1 [86]. However, a high amount of uric acid released by tumor cells after chemotherapy mainly occurs in hematological malignancies rather than solid tumors. Consequently, if NALP3 inflammasome and IL-1 are involved in the immunogenicity of chemotherapy-induced cell death in solid tumor, the activator of inflammasome may not be uric acid.
Conclusions
Nowadays, oncologists mainly give credit to anticancer drugs, which harbor the strongest antiproliferative effects against tumor cells, based on the assumption that these drugs only act via cell-autonomous effects. Emerging concept focuses on the so-called “targeted therapies”, although targeted therapies often trigger many signaling pathways and act through many mechanisms [87]. The discovery that even “targeted therapies” can have some surprising bystander effects offer a new avenue of investigations of these bystander effects for all cancer therapies included cytotoxic drugs.
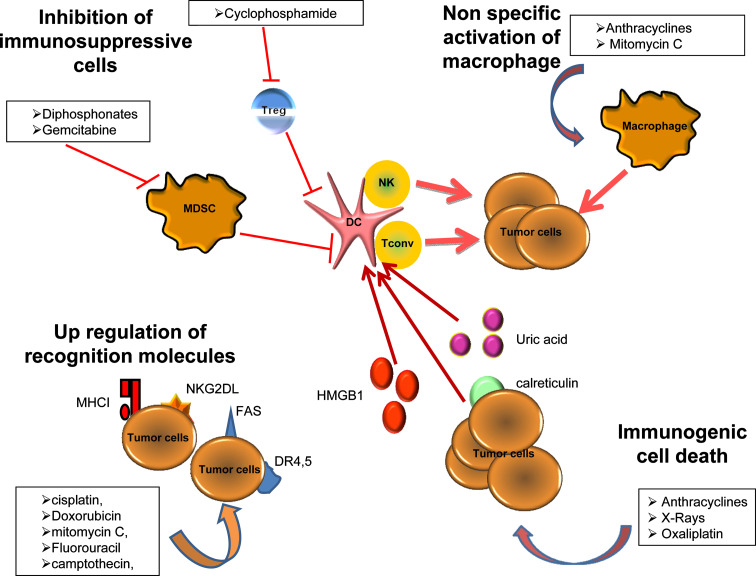
Bodies of evidence in literature suggest that, in many setting, cytotoxic drugs positively influence the immune system (Fig. 1). This side effect on the tumor immunology modifies the clinical response to chemotherapy. We demonstrated that in breast cancer patients, a constitutive deficiency of the immune system—that is, a loss of function polymorphism of TLR4—blunts the antitumor effect of anthracyclines in an adjuvant setting [77]. Moreover, we also highlight that T cell infiltration is modified by neoadjuvant chemotherapy in breast cancer patients. Indeed, a good response to chemotherapy is significantly associated with infiltration of cytotoxic T cells and disappearance of Treg in the tumor bed, suggesting that chemotherapy efficacy may partially rely on its immunological effect. Altogether, these data strongly suggest that the host immune system reaction to chemotherapy makes a decisive contribution to the efficacy of anticancer cytotoxic therapies [88]. In human colon cancer, gemcitabine, a drug that can eliminate MdSC, and oxaliplatin, a drug that can induce an immunogenic cell death, may be efficiently combined with an immunotherapy associating granulocyte macrophage colony-stimulating factor and interleukin-2. Such therapies induce very high objective response and disease control rates with a significant reduction in immunosuppressive regulatory T cells [89, 90].
Fig. 1.
Multimodal effects of chemotherapeutic agents on the immune system
In the future, selection of more efficient cancer treatments might rely on associations of cytotoxic drugs, which allow elimination of immunosuppressive cells, with trigger activation of effector components of the immune system. Such therapies could then be given alone or in association with a tumor vaccine.
References
- 1.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 2.Ferraro C, Quemeneur L, Prigent AF, Taverne C, Revillard JP, Bonnefoy-Berard N. Anthracyclines trigger apoptosis of both G0–G1 and cycling peripheral blood lymphocytes and induce massive deletion of mature T and B cells. Cancer Res. 2000;60:1901–1907. [PubMed] [Google Scholar]
- 3.Genestier L, Paillot R, Fournel S, Ferraro C, Miossec P, Revillard JP. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J Clin Invest. 1998;102:322–328. doi: 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Anaissie EJ, Kontoyiannis DP, O’Brien S, et al. Infections in patients with chronic lymphocytic leukemia treated with fludarabine. Ann Inter Med. 1998;129:559–566. doi: 10.7326/0003-4819-129-7-199810010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Weinblatt ME, Coblyn JS, Fox DA, et al. Efficacy of low-dose methotrexate in rheumatoid arthritis. NEJM. 1985;312:818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL, Cohen JA. Treatment of multiple sclerosis with cyclophosphamide: critical review of clinical and immunologic effects. Mult Scler. 2002;8:142–154. doi: 10.1191/1352458502ms790oa. [DOI] [PubMed] [Google Scholar]
- 8.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 9.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko HJ, Kim YJ, Kim YS, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 11.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North RJ, Dye ES. Ly 1 + 2- suppressor T cells down-regulate the generation of Ly 1–2 + effector T cells during progressive growth of the P815 mastocytoma. Immunology. 1985;54:47–56. [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 14.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 17.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 18.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 19.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JY, Zhang XS, Ding Y, et al. The changes of CD4+ CD25+/CD4+ proportion in spleen of tumor-bearing BALB/c mice. J Transl Med. 2005;3:5. doi: 10.1186/1479-5876-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu P, Lee Y, Liu W, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Andaloussi A, Lesniak MS. CD4+ CD25+ FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neurooncol. 2007;83:145–152. doi: 10.1007/s11060-006-9314-y. [DOI] [PubMed] [Google Scholar]
- 23.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3 + regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 24.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+ CD25+ Foxp3+ regulatory T cells. J Immunol. 2006;177:6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 26.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermol Sci. 2005;39:105–112. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+) 25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 28.Motoyoshi Y, Kaminoda K, Saitoh O, et al. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16:141–146. [PubMed] [Google Scholar]
- 29.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JY, Wu Y, Zhang XS, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghiringhelli F, Menard C, Terme M, et al. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasprowicz DJ, Droin N, Soper DM, Ramsdell F, Green DR, Ziegler SF. Dynamic regulation of FoxP3 expression controls the balance between CD4+ T cell activation and cell death. Eur J Immunol. 2005;35:3424–3432. doi: 10.1002/eji.200526339. [DOI] [PubMed] [Google Scholar]
- 34.Taieb J, Chaput N, Schartz N, et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 35.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor–inducer T-cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 36.Berd D, Maguire HC, Jr, McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol. 1990;8:1858–1867. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- 37.Jones RC, Kelley M, Gupta RK, et al. Immune response to polyvalent melanoma cell vaccine in AJCC stage III melanoma: an immunologic survival model. Ann Surg Oncol. 1996;3:437–445. doi: 10.1007/BF02305761. [DOI] [PubMed] [Google Scholar]
- 38.Livingston PO, Wong GY, Adluri S, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 39.Miller K, Abeles G, Oratz R, et al. Improved survival of patients with melanoma with an antibody response to immunization to a polyvalent melanoma vaccine. Cancer. 1995;75:495–502. doi: 10.1002/1097-0142(19950115)75:2<495::AID-CNCR2820750212>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell MS, Von Eschen KB. Phase III trial of Melacine melanoma theraccine versus combination chemotherapy in the treatment of stage IV melanoma (Meeting abstract) Proc Am Soc Clin Oncol. 1997;16:494. [Google Scholar]
- 41.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Colleoni M, Orlando L, Sanna G, et al. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: antitumor activity and biological effects. Ann Oncol. 2006;17:232–238. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 43.Samaritani R, Corrado G, Vizza E, Sbiroli C. Cyclophosphamide “metronomic” chemotherapy for palliative treatment of a young patient with advanced epithelial ovarian cancer. BMC Cancer. 2007;7:65. doi: 10.1186/1471-2407-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munoz R, Shaked Y, Bertolini F, Emmenegger U, Man S, Kerbel RS. Anti-angiogenic treatment of breast cancer using metronomic low-dose chemotherapy. Breast. 2005;14:466–479. doi: 10.1016/j.breast.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Wong MK, Yi H, Watkins S, Laird AD, Wolf SF, Gorelick E. Combined therapy of local and metastatic 4T1 breast tumor in mice using SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and the immunostimulator B7.2-IgG fusion protein. Cancer Res. 2002;62:5727–5735. [PubMed] [Google Scholar]
- 46.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–8413. [PubMed] [Google Scholar]
- 47.Borg C, Terme M, Taieb J, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest. 2004;114:379–388. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature dendritic cells into TGF-b secreting cells inducing CD4+ CD25+ regulatory T cells proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1 +/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 53.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 55.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nature Rev. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 56.Chang BD, Broude EV, Dokmanovic M, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–3767. [PubMed] [Google Scholar]
- 57.Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/S0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Wong SC, Pan J, et al. Evidence of cisplatin-induced senescent-like growth arrest in nasopharyngeal carcinoma cells. Cancer Res. 1998;58:5019–5022. [PubMed] [Google Scholar]
- 59.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacour S, Hammann A, Wotawa A, Corcos L, Solary E, Dimanche-Boitrel MT. Anticancer agents sensitize tumor cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated caspase-8 activation and apoptosis. Cancer Res. 2001;61:1645–1651. [PubMed] [Google Scholar]
- 62.Micheau O, Solary E, Hammann A, Martin F, Dimanche-Boitrel MT. Sensitization of cancer cells treated with cytotoxic drugs to fas-mediated cytotoxicity. J Natl Cancer Inst. 1997;89:783–789. doi: 10.1093/jnci/89.11.783. [DOI] [PubMed] [Google Scholar]
- 63.Haskill JS. Adriamycin-activated macrophages as tumor growth inhibitors. Cancer Res. 1981;41:3852–3856. [PubMed] [Google Scholar]
- 64.Martin F, Caignard A, Olsson O, Jeannin JF, Leclerc A. Tumoricidal effect of macrophages exposed to adriamycin in vivo or in vitro. Cancer Res. 1982;42:3851–3857. [PubMed] [Google Scholar]
- 65.Ogura T, Shindo H, Shinzato O, et al. In vitro tumor cell killing by peritoneal macrophages from mitomycin C-treated rats. Cancer Immunol Immunother. 1982;13:112–117. doi: 10.1007/BF00205310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shindo H, Ogura T, Masuno T, Hayashi S, Kishimoto S. Induction of activated macrophages by intraperitoneal injection of mitomycin C in mice. Cancer Immunol Immunother. 1985;20:145–150. doi: 10.1007/BF00205681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ujhazy P, Zaleskis G, Mihich E, Ehrke MJ, Berleth ES. Doxorubicin induces specific immune functions and cytokine expression in peritoneal cells. Cancer Immunol Immunother. 2003;52:463–472. doi: 10.1007/s00262-003-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mihich E. Anticancer drug-induced immunomodulation and cancer therapeutics. Curr Ca Ther Rev. 2007;3:174–193. doi: 10.2174/157339407781368350. [DOI] [Google Scholar]
- 69.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 70.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 71.Skoberne M, Beignon AS, Larsson M, Bhardwaj N. Apoptotic cells at the crossroads of tolerance and immunity. Curr Top Microbiol Immunol. 2005;289:259–292. doi: 10.1007/3-540-27320-4_12. [DOI] [PubMed] [Google Scholar]
- 72.Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 74.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 75.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 76.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 77.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 78.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 79.Shiratsuchi H, Basson MD. Extracellular pressure stimulates macrophage phagocytosis by inhibiting a pathway involving FAK and ERK. Am J Physiol. 2004;286:C1358–C1366. doi: 10.1152/ajpcell.00553.2003. [DOI] [PubMed] [Google Scholar]
- 80.Lake RA, Robinson BW. Immunotherapy and chemotherapy-a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 81.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::AID-IMMU2448>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 82.Wallace A, LaRosa DF, Kapoor V, Sun J, Cheng G, Jassar A, Blouin A, Ching LM, Albelda SM. The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes. Cancer Res. 2007;67:7011–7019. doi: 10.1158/0008-5472.CAN-06-3757. [DOI] [PubMed] [Google Scholar]
- 83.Baguley BC. Antivascular therapy of cancer DMXAA. Lancet Oncol. 2003;4:141–148. doi: 10.1016/S1470-2045(03)01018-0. [DOI] [PubMed] [Google Scholar]
- 84.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 85.Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64:5059–5062. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 86.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 87.Johnson BF, Clay TM, Hobeika AC, Lyerly HK, Morse MA. Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Exp Opin Biol Ther. 2007;7:449–460. doi: 10.1517/14712598.7.4.449. [DOI] [PubMed] [Google Scholar]
- 88.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F (2008) Pathological complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor infiltrating Foxp3+ regulatory T cells. Clin Cancer Res 14 (in press) [DOI] [PubMed]
- 89.Correale P, Cusi MG, Del Vecchio MT, Aquino A, Prete SP, Tsang KY, Micheli L, Nencini C, La Placa M, Montagnani F, Terrosi C, Caraglia M, Formica V, Giorgi G, Bonmassar E, Francini G. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol. 2005;175:820–828. doi: 10.4049/jimmunol.175.2.820. [DOI] [PubMed] [Google Scholar]
- 90.Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini C, Ferrari F, Giorgi G, Bonmassar E, Francini G. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]