Abstract
T cell targeting immunotherapy is now considered a possible strategy in acute myelogenous leukaemia (AML), and IFNγ release may then contribute to the antileukaemic effects. We investigated the effects of IFNγ on native human AML cells. Normal T cells could be activated to release IFNγ in the presence of AML cells. Furthermore, high levels of CD119 (IFNγ receptor α chain) expression were observed for all 39 patients examined. Receptor expression was decreased after exposure to exogenous IFNγ, and receptor ligation caused Stat1 phosphorylation but no phosphorylation of the alternative messengers Erk1/2. The effect of exogenous IFNγ on AML blast proliferation was dependent on the local cytokine network and IFNγ (1) inhibited proliferation in the presence of exogenous IL1β, GM-CSF, G-CSF and SCF; (2) had divergent effects in the presence of IL3 and Flt3 (65 patients examined); (3) inhibited proliferation in the presence of endothelial cells but had divergent effects in the presence of fibroblasts, osteoblasts and normal stromal cells (65 patients examined). IFNγ increased stress-induced (spontaneous) in vitro apoptosis as well as cytarabine-induced apoptosis only for a subset of patients. Furthermore, IFNγ decreased the release of proangiogenic CXCL8 and increased the release of antiangiogenic CXCL9–11. We conclude that IFNγ can be released in the presence of native human AML cells and affect AML cell proliferation, regulation of apoptosis and the balance between pro- and antiangiogenic chemokine release.
Keywords: Acute myeloid leukaemia, Interferon gamma, Cytokines, Apoptosis, Proliferation
Introduction
Interferon gamma (IFNγ) (reviewed in [1, 2]) is a type II interferon synthesized by T lymphocytes and natural killer cells. It has pleiotropic effects on a wide range of target cells. Among the important effects of IFNγ are (1) upregulation of MHC class I and induction of MHC class II protein expression by a variety of immunocompetent as well as nonimmune cells; (2) regulation of humoral immune responses; and (3) modulation of the release of a variety of other immunomodulatory cytokines such as IL12 and tumour necrosis factor (TNF) α. IFNγ-induced gene expression is important for several of these effects, but the molecular mechanisms have not been characterized in detail.
Acute myelogenous leukaemia (AML) is an aggressive malignancy characterized by accumulation of immature myeloid cells [3]. The overall disease-free survival is less than 50% even for younger patients who can receive the most intensive therapy [3]. However, the experience from allotransplanted patients shows that T cells can mediate posttransplant anti-AML effects and thereby contribute to improved prognosis. Targeting of autologous T cells is therefore considered a possible therapeutic approach [4, 5]. T cell release of antiproliferative, proapoptotic or antiangiogenic cytokines is then a possible antileukaemic effector mechanism, and IFNγ is of particular interest because it is released at high levels by human T cells. These high levels have been observed both for normals, AML patients receiving intensive chemotherapy and allogeneic stem cell recipients [6, 7]. IFNγ has also been tried in the treatment of AML, and the results from these initial small clinical studies suggest that IFNγ can mediate antileukaemic effects in vivo [8, 9].
The heterodimeric IFNγR (CD119) complex (reviewed in [2, 10]) consists of the IFNγ-binding R1 or α chain (CDw119) and the signal-transducing R2 or β chain. Receptor ligation induces phosphorylation of Janus kinases 1 and 2 that mediate activation of signal transducer and activator of transcription (STAT) molecules as well as Stat1-independent pathways [2, 10]. In a recent study we demonstrated that downstream intracellular signalling events after ligation of CD119 can even be used in prognostic classification of AML patients [11]. In vitro effects of IFNγ on native human AML cells have also been investigated previously, but in these studies (1) relatively low numbers of patients have been included; (2) patient selection has not been described; (3) the clinical and biological characterization of the patients are incomplete; and (4) only the examination of proliferation and membrane molecule expression have been included [9, 12–18]. In the present study we therefore characterized CD119 receptor expression, intracellular events following CD119 ligation and a wide range of functional effects of IFNγ in native human AML derived from a large group of consecutive patients.
Materials and methods
Acute leukaemia patients
The study was approved by the local Ethics Committee and samples collected after informed consent.
Acute leukaemia cells were derived from 87 consecutive AML patients (39 females and 48 males; median age 63 years with range 26–84 years) and 9 acute lymphoblastic leukaemia (ALL) patients [19] with high peripheral blood blast counts. Sixty-nine patients had de novo AML, the remaining minority had AML relapse (4 patients), chronic myeloid leukaemia in blast phase (2 patients) or AML secondary to chemotherapy or primary myelodysplasia (4 and 12 patients, respectively). The patients showed the following FAB classification: M0/M1 24 patients, M2/M3 28 patients, M4/M5 34 patients and M6 1 patient. Forty-six patients had >20% CD34+ blasts. Cytogenetic analysis was performed for 63 patients and the abnormalities classified as described by Wheatley et al. [20]; 38 patients had normal chromosomes whereas 5 patients had low-risk, 10 patients high-risk and 10 patients intermediate-risk abnormalities. Seventy-eight patients were tested for genetic Flt3 abnormalities [21]; 27 patients had internal tandem duplications and 9 patients D835 mutations.
Nine ALL patients were examined (five females and four males; median age 24 years with range 18–74 years). One patient had T ALL, and eight had B ALL (three pro-ALL, two pre-ALL, three common B ALL).
Preparation of native human leukaemia blasts
Leukaemic peripheral blood mononuclear cells (PBMC) were isolated by density gradient separation (Ficoll-Hypaque; NyCoMed, Oslo, Norway; specific density 1.077) from the peripheral blood of patients with >80% of AML blasts among blood leukocytes. Cells were stored frozen in liquid nitrogen [21]. The percentage of blasts among leukaemic PBMC generally exceeded 95% [21, 22], the contaminating cells being mainly small lymphocytes.
Nonleukaemic cells
Endothelial cells
Human lung microvascular endothelial cells were obtained as frozen vials (Cambrex Bio Science Walkersville, Walkersville, MD, USA) and stored in liquid nitrogen until used. The cells were derived from a healthy 16-year-old white male (product code CC-2527, lot no. 3F1056). These cells (1) showed a doubling time in culture of approximately 18; (2) stained positive for acetylated LDL uptake stain, factor VIII related antigen and PECAM staining; (3) stained negative for alpha actin expression; and (4) were negative when tested for mycoplasma, human immunodeficiency virus 1, hepatitis B and hepatitis C (polymerase chain reactions) (distributor’s information).
Human fibroblasts
The cell line HFL1 (ATCC, Vanessa, no. CCL-153) was derived from the lungs of a 16 to 18-week-old fetus, has a typical adherent growth pattern and a diploid karyotype (distributor’s information). Its functional characteristics have been described previously [23–25].
Human osteoblasts
The cell line Cal72 (Deutsche Sammlung von Zellkulturen und Mikroorganismen, Braunschwaig, Germany) has previously been characterized in detail [25–28]. It has a phenotype close to normal osteoblasts with an adherent growth pattern and a broad cytokine release profile [21].
Normal human bone marrow stromal cells
These cells were delivered in frozen vials (Cambrex) and stored in liquid nitrogen until use in the coculture assay. The cells were derived from a healthy 20-year-old white female. Bone marrow mononuclear cells were then derived by gradient separation (specific density 1.077) and cultured in Myelocult growth medium (BioWhitacker) for 4 weeks. The stromal cells represent the adherent cell population of the cultured cells and are a heterogeneous population of fibroblasts, reticulum cells, endothelial cells, macrophages and fat cells. The cells showed a purity of 95% and tested negative for mycoplasma, human immunodeficiency virus 1, hepatitis B and hepatitis C (polymerase chain reaction) (distributor’s information).
Flow cytometric analysis of membrane molecule expression
Membrane molecule expression was analysed by flow cytometry (FCM) using either PE, FITC or APC-conjugated anti-CD119, HLA-DR, CD40, CD80 and CD83 monoclonal antibodies (Becton Dickinson, San Jose, USA). The cut-off for positive cells was defined as a fluorescence corresponding to 1% positive cells when using an isotypic control antibody.
Analysis of STAT and Erk1/2 phosphorylation in AML cells
The method has been described in detail previously [11]. Briefly, AML blasts were incubated for 15 min with or without IFNγ in medium. STAT and Erk1/2 phosphorylation was analysed by FCM using Alexa647-conjugated anti-phospho-Stat1 (pY701), anti-phospho-Stat5 (pY694), anti-phospho-Erk1/2 (T202/Y204) and Alexa488-conjugated anti-phospho-Stat3 (pY705) monoclonal antibodies (BD, Biosciences, Norway).
In vitro culture of native human AML blasts
Reagents
Unless otherwise stated the culture medium Stem Span SFEM™ (StemSpan; Stem Cell Technologies, Inc., Vancouver, BC, Canada) supplemented with 100 μg/ml of gentamycin was used for culture of AML cells. In cocultures containing nonleukaemic cells the medium was also supplemented with 10% heat-inactivated fetal calf serum (BioWhitacker) [25]. Cultures containing endothelial cells were prepared in the endothelial cell EBM-2 medium supplemented with EGM-2MV single quots (Cambrex Biosciences); preliminary experiments demonstrated that this medium could also be used for in vitro culture of AML blasts. The following exogenous cytokines were used at 50 ng/ml: IFNγ, TNFα, IL1RA, IL1β, IL3, IL4, IL6, IL8, IL10, IL13, Flt3L, SCF, GM-CSF and G-CSF (PeproTech EC Ltd, London, UK). The cytotoxic drug idarubicin (Zavedos, Pfizer, Inc., NY, USA) was used at 0.1 and 0.01 μM, while cytarabine (Pfizer, Inc.) was used at 1 and 0.1 μM concentrations.
Proliferation in suspension cultures
As described previously [28], 5×104 cells/well were cultured in 150 μl medium in flat-bottomed microtiter plates (Costar 3796; Cambridge, MA, USA). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2. After 6 days 20 μl of 3H-thymidine (37 kBq/well; TRA 310, Amersham International, Amersham, UK) in 0.9% NaCl solution was added to each well and nuclear radioactivity assayed 18 h later by liquid scintillation counting.
Coculture with nonleukaemic cells
Cultures were prepared in transwell culture plates (Transwell 3401; Costar) where cells in the lower large compartment were separated from the cells in the upper small chamber of the same well by a semipermeable membrane with pore diameter of 0.4 μm [29]. Nonleukaemic cells were seeded in the lower compartment (104 cells in 1 ml). Leukaemia blasts (106 cells in 0.5 ml) were added to the upper chamber and the cultures thereafter incubated for 7 days. Cultures were always ended before the nonleukaemic cells were confluent.
Analysis of cell proliferation
Cultures were prepared as described above and incubated for 6 days before 3H-thymidine (280 kBq/well in 150 μl saline) was added and cultures incubated for an additional 18 h. The leukaemic cells were then resuspended and nuclear radioactivity assayed for 50 μl aliquots by liquid scintillation counting. The adherent nonleukaemic cells were washed in isotonic saline before 300 μl/well of trypsin–EDTA solution (Stem Cell Technologies) was added and nuclear radioactivity assayed in 70 μl aliquots.
T cell activation in the presence of cocultured AML and bone marrow stromal cells
The cultures were prepared in transwell cultures (Costar 3401 Transwell plates). Native human AML cells (1×106 cells) were cultured in the lower chamber in direct contact with 1×104 normal bone marrow stromal cells. Normal PBMC derived from a healthy individual (5×104 cells) were incubated in the upper chamber and activated by anti-CD3 antibody (Central Laboratory of the Netherlands’ Red Cross Blood Transfusion Services, Amsterdam, The Netherlands, final dilution 1:500) [30]. The cultures were incubated for 4 days before IFNγ levels were determined.
Analysis of AML cell viability during in vitro culture
AML blasts were incubated for 24 and 48 h (24 well Costar 3524 culture plates; 2×106 cells in 2 ml Stem Span™ medium per well) before the percentages of viable/apoptotic/necrotic cells were determined by FCM analysis of AnnexinV-FITC and propidium iodide positive cells (Nexins Research, Kattendjike, The Netherlands) [24].
Analysis of AML cell cytokine secretion
As described previously [28], 1×106 AML blasts/ml were cultured in 24 wells tissue culture plates (Costar 3524; 2 ml cell suspension/well) for 48 h before supernatants were harvested. ELISA analyses were used to determine the levels of IL1β, IL6, TNFα (Pelikine compact ELISA kits; Central Laboratory of the Netherlands’ Red Cross Blood Transfusion Services), CXCL8–11, G-CSF and GM-CSF (Quantikine ELISA kits; R&D Systems) in the supernatants. The minimal detectable levels were IL1β 0.8 pg/ml, IL6 0.8 pg/ml, TNFα 1.0 pg/ml, CXCL8 3.5 pg/ml, CXCL9 3.8 pg/ml, CXCL10 1.7 pg/ml, CXCL11 13.9 pg/ml, GM-CSF 3 pg/ml and G-CSF 8 pg/ml.
Presentation of the data
Cell proliferation was assayed by 3H-thymidine incorporation and the mean counts per minute (cpm) of triplicate determinations were used in all calculations. Detectable 3H-thymidine incorporation was defined as >1,000 cpm. A significant alteration of 3H-thymidine incorporation was defined as a difference corresponding to (1) an absolute value of at least 2,000 cpm and (2) this absolute value being >20% of the corresponding control. For statistical analysis the Wilcoxon’s signed rank test was performed. Differences were regarded as significant when P<0.05 with a two-sided test.
Results
IFNγ is released by T cells activated in the presence of native human AML cells and bone marrow stromal cells
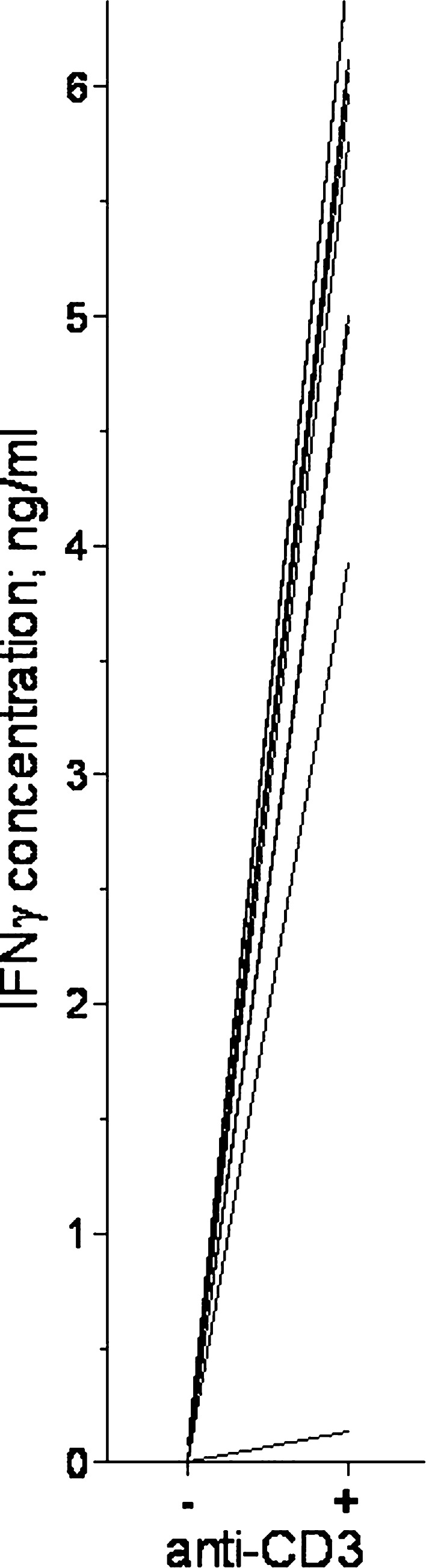
Native human AML cells are characterized by constitutive release of several immunoregulatory cytokines that can have antigen-nonspecific effects on the release of IFNγ by activated T cells [31]. This constitutive release differs between patients [31]. To investigate whether IFNγ release by normal T cells could take place in the presence of this leukaemia-determined cytokine network, AML cells derived from 14 consecutive patients were cultured together with leukaemia-supporting bone marrow stromal cells in the lower chamber of transwell cultures, and normal PBMC derived from a healthy individual were incubated in the upper chamber. The cultures were prepared in Stem Span™ medium with and without anti-CD3 MoAb as the T cell activating signal. High IFNγ levels were detected in the supernatants for anti-CD3-stimulated PBMC in the presence of AML cells and stromal cells (exceeding 2,000 pg/ml for all patients but one), whereas low levels (<100 pg/ml) were observed for control cultures without T cells (Fig. 1). Thus, T cells can be activated to release high levels of IFNγ in the presence of native human AML cells.
Fig. 1.
IFNγ concentrations in culture supernatants when normal T cells were activated with anti-CD3 in the presence of AML cells and BMSC. Normal PBMC were cultured in the upper chamber and BMSC + AML cells in the lower chamber of transwell cultures. AML cells were derived from 14 consecutive patients. Cultures were prepared either in medium alone (−) or with (+) anti-CD3, and supernatants were collected after 4 days. IFNγ levels were determined by ELISA analysis (P=0.0002). Cultures with AML cells or PBMC alone showed undetectable IFNγ levels
Previous studies have demonstrated that IFNγ can increase the AML cell expression of several membrane molecules involved in immune recognition, including HLA-class II (especially HLA-DR), CD40, CD54, CD80 and possibly CD83 [32, 33]. We therefore investigated whether similar alterations could be observed when native human AML cells were cultured together with normal, activated, IFNγ-secreting T cells. Leukaemia cells derived from nine consecutive patients were cultured in the lower chamber of transwell cultures and anti-CD3-stimulated normal T cells in the upper chamber. Membrane molecule expression was compared after 4 days for cocultured AML cells and cells incubated in medium alone. The percentages of HLA-DR+ AML cells were relatively high for both cells cultured with and without T cells (median 65 and 82%, respectively), but the mean fluorescence intensity (MFI) was significantly increased for cocultured cells (median 21, variation range 0–50) compared with cells incubated in medium alone (median 59, range 12–131; P=0.011). Coculture did not alter CD40, CD80 and CD83 expressions (all cultures showing <10% positive cells).
CD119 (IFNγ receptor) is expressed by native human AML cells
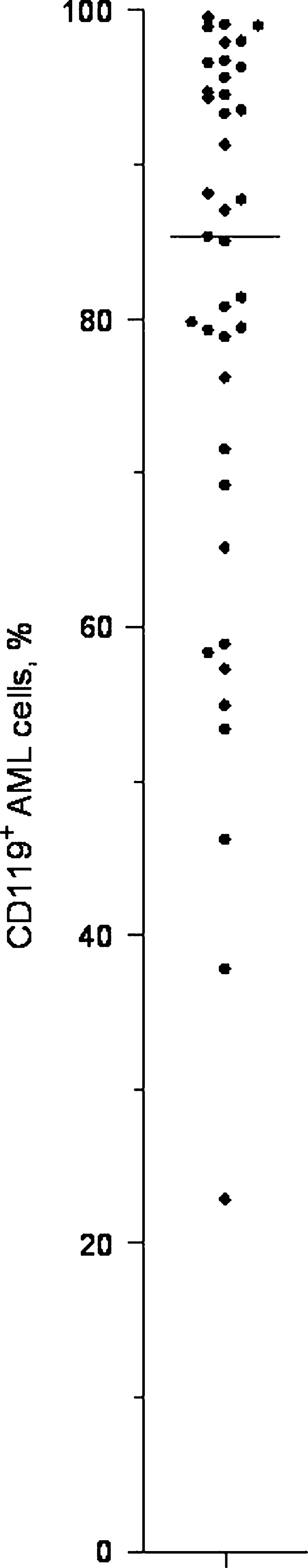
The expression of CD119 was examined for AML blasts derived from 39 randomly selected patients (Fig. 2). CD119 expression was detected for all patients (median percentage of positive cells 85%, variation range 22–99%), and more than 70% CD119+ cells were observed for 29 out of the 39 patients. High expression was observed independent of morphological signs of differentiation (FAB classification), cytogenetic abnormalities or Flt3 mutations. For all patients, CD119 positivity showed a uniform distribution with no evidence for dual populations. The percentage of positive cells showed a significant correlation with MFI (Pearson correlation; r=0.64; P<0.01), but the CD119 expression also varied for patients with a high percentage of CD119+ cells (i.e. the MFI varied with a factor of 2.1 for AML cell populations with more than 95% CD119+ cells).
Fig. 2.
Expression of CD119 (IFNγ-receptor α chain) by native human AML cells. CD119 expression was examined by flow cytometry for 39 randomly selected patients. The results are expressed as the percentage of positive cells. Median is denoted in the figure
CD119 (IFNγ receptor) expression by AML cells decreases after exposure to IFNγ
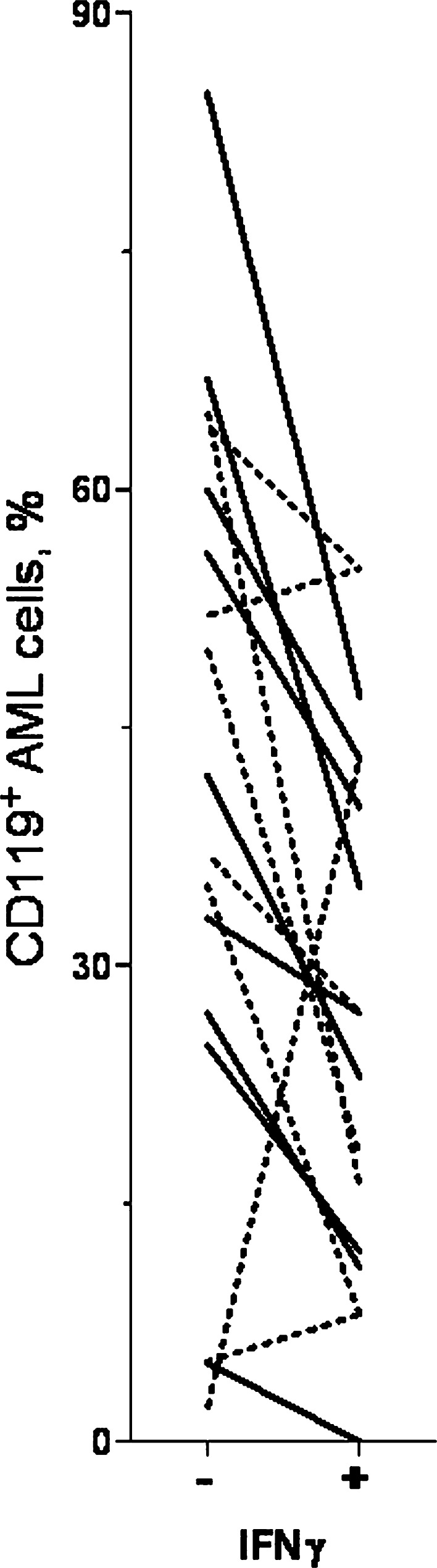
AML cells derived from nine consecutive patients were cultured in vitro and CD119 expression compared for AML cells cultured in medium alone and leukaemia cells exposed to IFNγ during culture by adding either (1) exogenous IFNγ 50 ng/ml to the medium or (2) coculture of AML cells with IFNγ-secreting anti-CD3-activated normal T cells (see above). CD119 expression was analysed after 24 and 72 h, respectively. CD119 expression was decreased both in the presence of exogenous IFNγ and after coculture (Fig. 3).
Fig. 3.
Effect of IFNγ on CD119 expression by in vitro cultured AML cells. Leukaemia cells were derived from nine consecutive patients and we compared CD119 expression for AML cells cultured in medium alone (−) and cells exposed to IFNγ (+) by either (1) adding exogenous IFNγ 50 ng/ml to the medium (solid line; 24 h culture, n=9); or (2) coculturing AML cells with anti-CD3-activated, IFNγ-secreting T cells (dashed line; 72 h culture, n=8). The results are presented as percentage of CD119+ cells
CD119 ligation increases Stat1 phosphorylation
The effects of IFNγ on STAT and Erk1/2 phosphorylation status were investigated for 11 randomly selected patients. We investigated Stat1, Stat3, Stat5 and Erk1/2, and the frequency of positive phosphorylated cells as well as MFI were determined for AML blasts incubated in vitro with or without 50 ng/ml IFNγ. Cells from eight patients were tested twice in independent experiments. Increased MFI of phospho-Stat1 was observed for all patients after incubation with IFNγ (median MFI 4.2, range 1.5–45.5) compared to medium alone (median MFI 1.1, range 0.5–18.7; P=0.002), whereas no significant effects on pStat3, pStat5 or pErk1/2 were observed (data not shown).
IFNγ affects spontaneous and cytokine-dependent in vitro AML cell proliferation
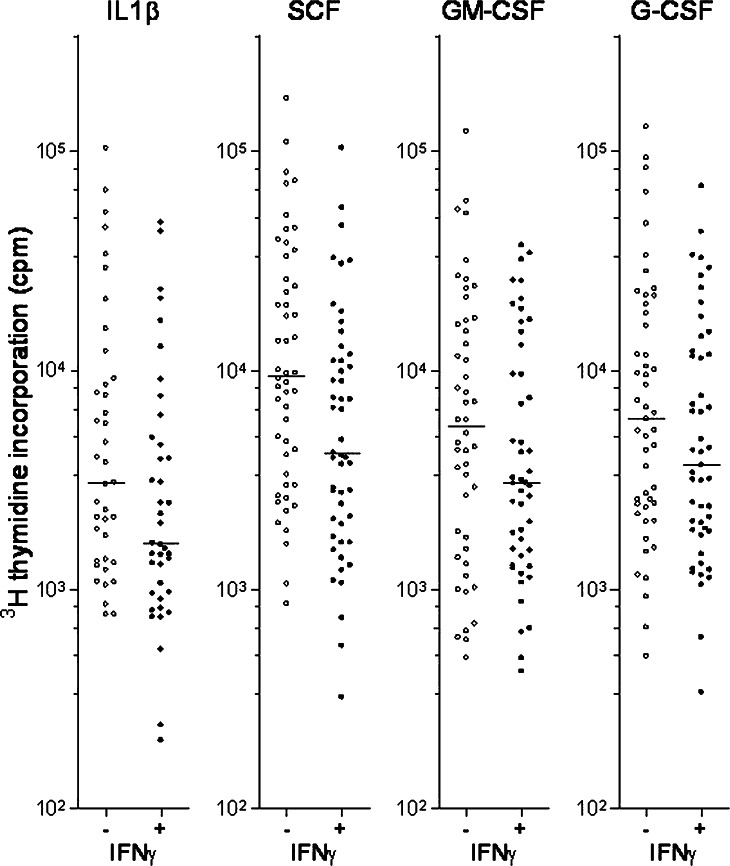
AML blasts derived from 65 consecutive patients were cultured in medium alone or in medium containing IFNγ 50 ng/ml. Detectable proliferation corresponding to at least 1,000 cpm was observed only for 21 patients. IFNγ caused no statistically significant alteration of spontaneous in vitro proliferation. AML blasts derived from the same patients were also cultured in the presence of exogenous cytokines, and 3H-thymidine incorporation was assayed after 7 days of culture. The effect of adding IFNγ was examined in the presence of 50 ng/ml of IL1β, IL3, Flt3L, SCF, GM-CSF or G-CSF. Our statistical analyses included the results only for those patients that showed detectable proliferation (corresponding to >1,000 cpm) for either the cytokine control or the corresponding IFNγ-containing culture. IFNγ caused a statistically significant inhibition of cytokine-dependent AML blast proliferation in the presence of IL1β, SCF, GM-CSF and G-CSF (Fig. 4, Table 1); for many of these patients the inhibitory effect exceeded 2,000 cpm and 20% of the IFNγ-free control. In contrast, only borderline significance was observed for IL3 and no significant effect for Flt3L (Table 1).
Fig. 4.
Effects of IFNγ in combination with exogenous growth factors on in vitro AML blast proliferation. AML blasts from 65 consecutive patients were cultured in the presence of either IL1β, SCF, GM-CSF or G-CSF. The figure compares the proliferation (3H-thymidine incorporation) for cultures with (+) or without (−) IFNγ (50 ng/ml). Only those patients showing detectable proliferation (>1,000 cpm) with or without IFNγ were included in each comparison. Line denotes the median. IFNγ inhibited cytokine-dependent proliferation (P=0.0013 for IL1β, P<0.0001 for SCF, P=0.0398 for GM-CSF, P=0.0149 for G-CSF)
Table 1.
The effect of IFNγ on cytokine-dependent proliferation by native human AML cells: a summary of the results for 67 consecutive patients
| Exogenous cytokine | Number of patients with detectable proliferation in IFNγ-free controlsa | Statistical comparison of proliferative response (mean cpm ± standard error) | Number of patients with >20% inhibitionb | ||
|---|---|---|---|---|---|
| Cultures without IFNγ | Cultures with IFNγ | P valuec | |||
| None | 21 | 5,885±1,376 | 9,692±3,456 | NS | – |
| IL1RA | 25 | 5,768±1,113 | 5,169±1,519 | NS | – |
| IL1β | 40 | 11,911±3,353 | 6,060±1,679 | 0.001 | 16/40 |
| IL3 | 49 | 17,543±3,661 | 12,048±2,015 | 0.052 | 22/49 |
| SCF | 50 | 22,491±4,553 | 11,078±2,510 | <0.0005 | 36/50 |
| Flt3L | 53 | 15,107±3,252 | 12,313±1,914 | NS | – |
| GM-CSF | 48 | 13,554±3,099 | 7,929±1,425 | 0.039 | 23/48 |
| G-CSF | 49 | 16,037±3,716 | 9,672±1,912 | 0.015 | 25/49 |
Native human AML cells were cultured in serum-free medium and proliferation assayed as 3H-thymidine incorporation after 7 days. The results are presented as counts per minute (cpm)
NS no significance
aA total of 67 consecutive patients were examined, but only those patients with detectable proliferation (>1,000 cpm) either in the IFNγ-containing or corresponding IFNγ-free control were included in the statistical analysis
bThe column indicates the number of patients who showed an IFNγ-induced alteration corresponding to at least 2,000 cpm and exceeding 20% of the control response
cThe two-tailed Wilcoxon’s signed rank test was used for the statistical analysis
IFNγ has divergent effects on AML blast apoptosis
The effect of IFNγ on AML blast apoptosis was investigated for 16 randomly selected patients. The percentages of viable/apoptotic/necrotic cells were determined after 24 and 48 h of in vitro culture with and without IFNγ, and for 10 patients the results were reproduced in independent experiments. The fraction of viable cells decreased gradually during culture and was accompanied by a corresponding increase in the percentage of apoptotic cells. The percentage of viable cells after 48 h showed a wide variation (median 46%, range 15–86%) and an inverse correlation with the percentage of apoptotic cells. IFNγ had reproducible divergent effects on the percentage of viable cells after 48 h of culture. An IFNγ-induced difference in cell viability exceeding 5% was observed for 8 of the 16 patients; increased viability was detected for 5 patients and decreased viability for 3 patients. The same divergence was observed after 24 h of culture, although the percentages of viable cells were generally higher both for cultures with and without IFNγ (data not shown).
Effects of IFNγ in combination with cytotoxic drugs on AML cell apoptosis and proliferation
We investigated the effect of IFNγ on AML cell apoptosis in the presence of cytarabine 0.1 and 1.0 μM. Ten consecutive patients were investigated, and the number of viable cells determined after 24 and 48 h. The overall results are summarized in Fig. 5. The presence of cytarabine decreased the percentage of viable cells, but the divergent effects of IFNγ on AML cell apoptosis was also detected in the presence of cytarabine. When analysing the overall results there was a significant correlation between IFNγ effects on apoptosis for cultures with and without cytarabine (Pearson correlation; r=0.76–0.90; P<0.02; for both concentrations and time points).
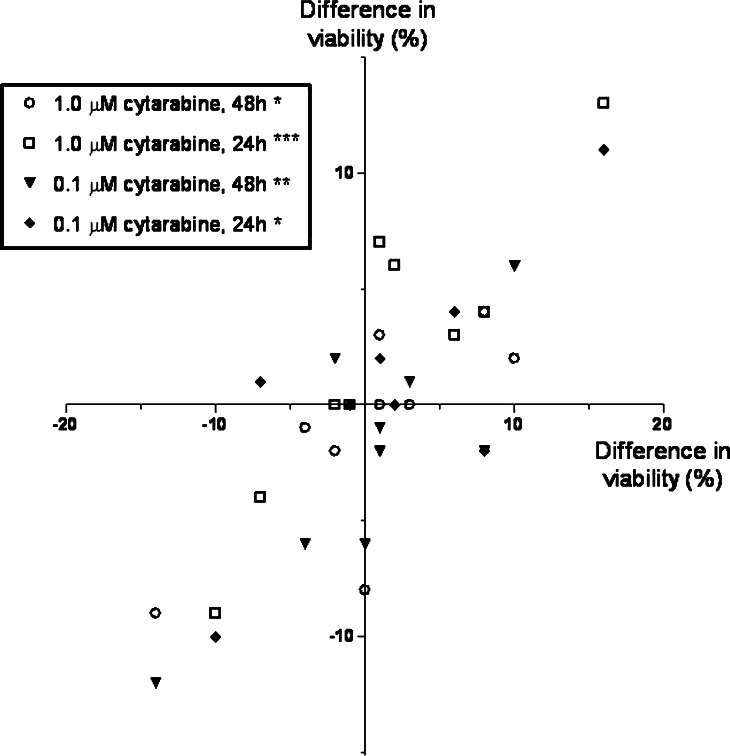
Fig. 5.
The effect of IFNγ on cytarabine-induced AML cell apoptosis. Leukaemia cells derived from 10 consecutive patients were examined. The median percentage of viable cells was 40% (variation range 17–84) after 24 h and 29% (range 17–83) after 48 h for cells cultured in medium alone, and viability was decreased both by cytarabine 1.0 μM (24 h: median viability 26%, range 18–57; 48 h: median 18; range 9–30) and 0.1 μM (24 h: median viability 37%, range 17–82; 48 h: median 25; range 17–80). The figure compares the effect of IFNγ 50 ng/ml on AML cell viability for cells cultured in the medium alone (horizontal axis) and medium with cytotoxic drugs (vertical axis). The results are presented as the difference in percentage of viable cells for cultures with and without IFNγ, and we present the overall results for both concentrations and both time intervals (*P=0.02; **P=0.008; ***P<0.001. Other symbols are explained in the figure)
We also investigated the effect of IFNγ on cytokine-dependent (GM-CSF + IL3 + SCF) AML cell proliferation (3H-thymidine incorporation) in the presence of cytarabine (0.1 and 1.0 μM) and idarubicin (0.01 and 0.1 μM). The same 10 consecutive patients were examined and the overall results showed that (1) IFNγ alone had divergent effects on cytokine-dependent AML cell proliferation; (2) both drugs inhibited AML cell proliferation and for the highest drug concentrations no detectable proliferation was observed for any patient; (3) the lower drug concentrations also decreased AML cell proliferation, and the presence of IFNγ then had divergent effects (data not shown).
Effects of IFNγ on the constitutive AML cell release of IL1β and GM-CSF
The levels of GM-CSF and IL1β were determined for AML cells derived from 65 consecutive patients, when cells were cultured in medium alone and together with IFNγ 50 ng/ml. Constitutive IL1β release was detected only for 14 patients (range 4–300 pg/ml) and GM-CSF release for 10 patients (range 3.1–2,313 pg/ml). IFNγ caused no significant alteration of these levels. In addition, the effect of IFNγ on AML cell proliferation was investigated when cells were cultured in the presence of exogenous IL1RA. IL1RA had no significant effect on the spontaneous AML blast proliferation in our in vitro model, and IFNγ did not alter blast proliferation in the presence of IL1RA (Table 1).
Effects of IFNγ on the constitutive AML cell release of angioregulatory chemokines
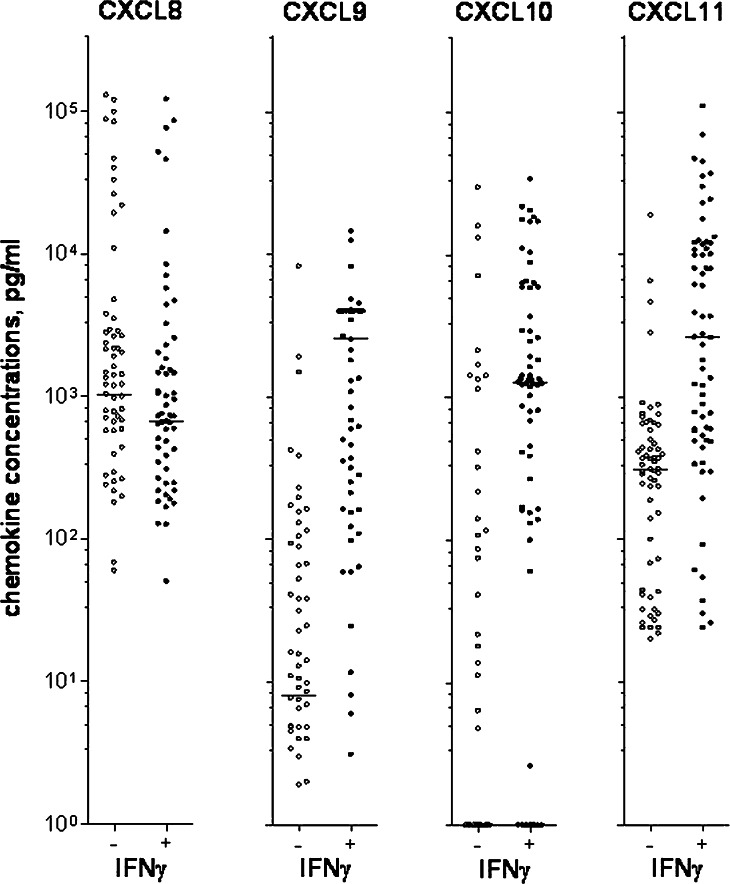
Angiogenesis is probably important both for leukemogenesis and chemosensitivity in AML, and the constitutive release of angioregulatory mediators by the leukaemia cells [34] is probably important for the local regulation of bone marrow angiogenesis in AML. We therefore investigated the effect of exogenous IFNγ on the constitutive release of pro- and antiangiogenic chemokines by AML cells. Leukaemia cells derived from 65 consecutive patients were cultured in vitro in medium alone or together with IFNγ for 48 h before the levels of angioregulatory chemokines were determined (Fig. 6). Constitutive release of proangiogenic CXCL8 was observed for most patients (57/67), and CXCL8 levels were slightly decreased in the presence of IFNγ (P=0.0004). In contrast, constitutive increased levels of antiangiogenic CXCL9 (P<0.0001), CXCL10 (P=0.0001) and CXCL11 (P<0.0001) were observed in the presence of IFNγ (Fig. 6). Our overall results thus suggest that IFNγ alters the balance between pro- and antiangiogenic chemokines in favour of antiangiogenic signalling, although high CXCL8 levels were still observed in the presence of exogenous IFNγ.
Fig. 6.
The effects of IFNγ on AML cell release of angioregulatory chemokines. The figure presents the proangiogenic CXCL8 and antiangiogenic CXCL9, CXCL10 and CXCL11 concentrations in supernatants for AML cells from 67 consecutive patients. The cells were cultured for 48 h either in medium alone (−) or in the presence of IFNγ 50 ng/ml (+). Chemokine levels were determined by ELISA analyses. Line denotes the median. IFNγ increased CXCL8 levels and decreased CXCL9–11 levels (P=0.0004 for CXCL8, P<0.0001 for CXCL9, P<0.0001 for CXCL10 and P<0.0001 for CXCL11)
Effects of IFNγ during coculture of native human AML cells and various stromal cells
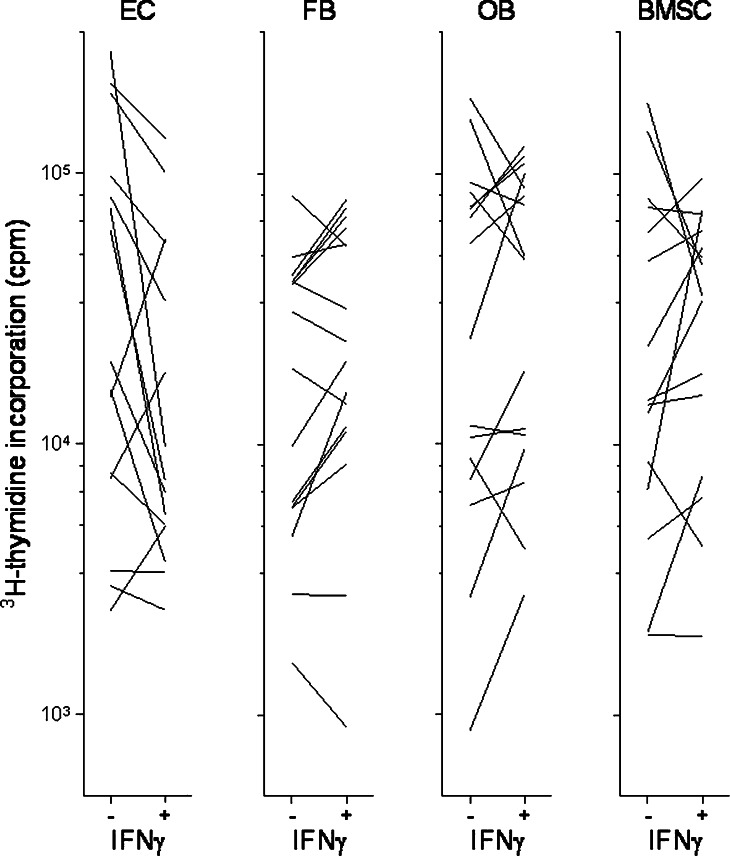
Angiogenesis is important in leukemogenesis [34], and the release of soluble mediators by microvascular endothelial cells has additional growth-enhancing and antiapoptotic effects on human AML cells (K.J. Hatfield, submitted). AML cells derived from 16 consecutive patients were cultured with and without IFNγ 50 ng/ml in transwell cultures together with microvascular endothelial cells. The proliferation of both cells was assayed as 3H-thymidine incorporation after 7 days. IFNγ caused a significant inhibition of AML cell proliferation in the presence of endothelial cells (Fig. 7, P=0.01). Furthermore, addition of IFNγ had divergent effects on endothelial cell proliferation compared to IFNγ-free controls: decreased endothelial cell proliferation was detected for 8 out of the 16 patients (Fig. 8). Thus, IFNγ may affect leukemogenesis indirectly through altered angioregulation.
Fig. 7.
Effect of IFNγ on in vitro proliferation of AML cells incubated in transwell culture together with either microvascular endothelial cells (EC), HFL1 fibroblasts (FB), Cal72 osteoblastic sarcoma cells (OB) or normal bone marrow stromal cells (BMSC). The figure compares leukaemia cell proliferation for cultures with (+) and without (−) IFNγ 50 ng/ml. Only those samples showing detectable proliferation (i.e. >1,000 cpm) for at least one of the in vitro models (+/−) are presented
Fig. 8.
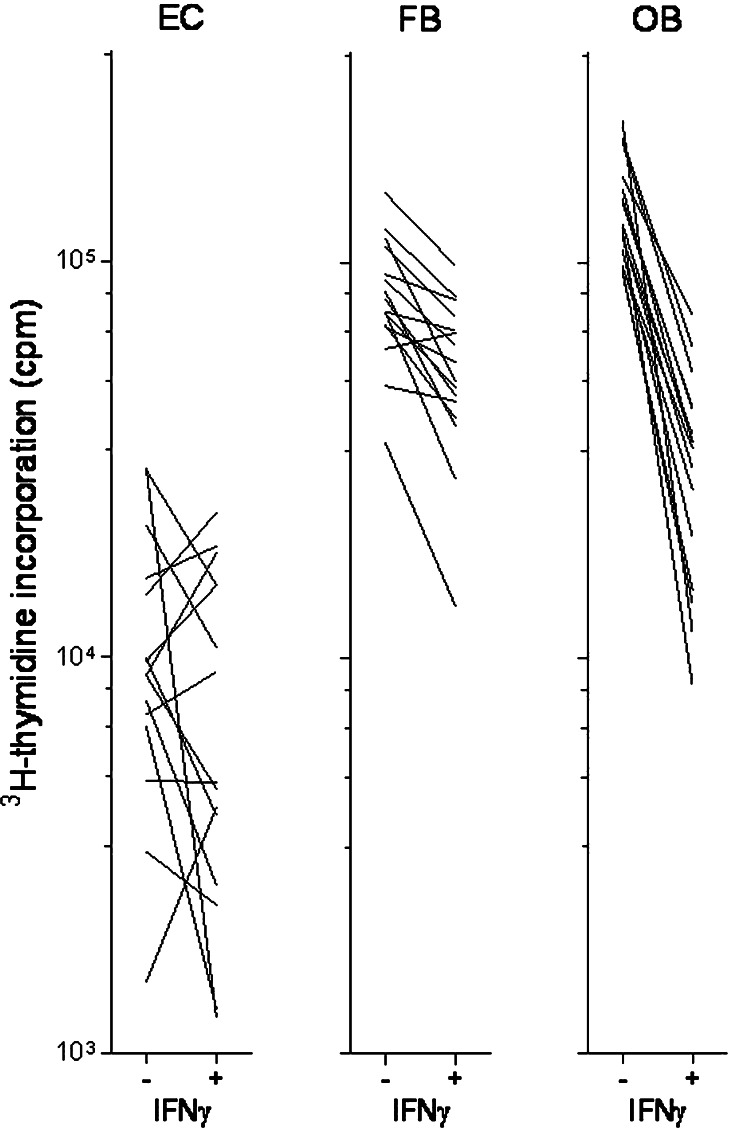
The effect of IFNγ on in vitro proliferation of nonleukaemic cells cocultured with AML blasts in transwell cultures. Microvascular endothelial cells (EC), HFL1 fibroblasts (FB) or Cal72 osteoblastic sarcoma cells (OB) were cultured together with AML blasts in the presence (+) or absence (−) of IFNγ 50 ng/ml. Only those patient samples showing detectable proliferation (i.e. >1,000 cpm) for at least one of the in vitro culture models (+/−) are presented
Various bone marrow stromal cells, including fibroblasts and osteoblast, have proliferation-enhancing and antiapoptotic effects on human AML cells [24, 25, 35–37]. AML cells derived from 16 consecutive patients were cocultured in transwell cultures together with fibroblasts (HFL1), osteoblastic sarcoma cells (Cal72) or normal bone marrow stromal cells. Cocultures were prepared with and without exogenous IFNγ and AML blast proliferation assayed after 7 days. IFNγ had divergent effects on AML blast proliferation during coculture with these nonleukaemic cells (Fig. 7). For the normal stromal cells this divergence was reproduced in repeated experiments. IFNγ inhibited the proliferation of cocultured Cal72 osteoblasts and HFL1 fibroblasts compared with cocultures without IFNγ (Fig. 8). Thus, IFNγ may affect leukemogenesis indirectly through effects on these nonleukaemic stromal cells.
Direct effects of IFNγ on nonleukaemic stromal cells
Low levels of CD119 expression were detected in microvascular endothelial cells (5% CD119+ cells), fibroblasts (6% CD119+ cells) and osteoblastic Cal72 cells (10% CD119+ cells). We therefore investigated the effect of exogenous IFNγ (50 ng/ml) on the proliferation of these cells. Cultures were prepared in medium alone and medium containing various exogenous growth factors (IL1β, IL3, IL6, IL8, GM-CSF, G-CSF, TNFα) and proliferation assayed by 3H-thymidine incorporation. Exogenous IFNγ did not alter the proliferation of endothelial cells, but IFNγ in combination with G-CSF, IL6 or IL8 increased the proliferation of these cells. Exogenous IFNγ caused a weak inhibition of Cal72 osteoblast proliferation and decreased HFL1 fibroblast proliferation by at least 70% both for cultures with and without exogenous cytokines.
Effects of IFNγ on cytokine-dependent proliferation by native human ALL blasts
Acute lymphoblastic leukaemia blasts derived from 12 consecutive patients were cultured in medium alone and in medium with various exogenous cytokines (IL4, IL10, IL13, SCF, GM-CSF, Flt3L) [38]. ALL cells did not proliferate in medium alone and for three patients detectable proliferation was not observed with any exogenous cytokine. Flt3L was the only cytokine that induced detectable proliferation for all the other nine patients, and IFNγ caused a strong inhibition of this proliferation for five patients (Table 2). Detectable proliferation was only observed for a minority of patients with the other cytokines, and either unaltered or decreased proliferation was then observed with IFNγ (data not shown).
Table 2.
The effect of IFNγ on Flt3L-dependent proliferation of native human ALL blasts
| Patient | IFNγ | Proliferative response |
|---|---|---|
| 1 | − | 32,335±1,888 |
| + | 251±64 | |
| 2 | − | 3,092±121 |
| + | 391±29 | |
| 3 | − | 819±135 |
| + | 1,511±722 | |
| 4 | − | 2,771±607 |
| + | 868±82 | |
| 5 | − | 1,959±89 |
| + | 2,346±73 | |
| 6 | − | 8,603±991 |
| + | 3,443±134 | |
| 7 | − | 5,388±328 |
| + | 2,967±214 | |
| 8 | − | 7,875±981 |
| + | 7,316±393 | |
| 9 | − | 1,137±334 |
| + | 1,467±422 |
ALL blasts were cultured for 7 days before proliferation was assayed as 3H-thymidine incorporation. The results are presented as the mean ± standard deviation of triplicate determinations. IFNγ was tested at 50 ng/ml. Results marked in bold represent a reduction exceeding 2,000 cpm or a reduction to undetectable levels
Discussion
IFNγ is a cytokine released by most CD4+ and CD8+ T cell clones, and this is true for healthy individuals, acute leukaemia patients with chemotherapy-induced cytopenia and bone marrow transplant recipients [6, 7]. IFNγ is released by T cells activated in vitro in the presence of native human AML cells [31, 39]. Taken together these experimental observations strongly suggest that local IFNγ release will be a part of antileukaemic T cell responses, and observations from clinical studies of IFNγ therapy have demonstrated that IFNγ has biological effects in vivo on human AML cells [8]. In this context we have investigated the effects of IFNγ on the functional characteristics of native human AML cells. Our results demonstrate that IFNγ can affect proliferation, cytokine release and regulation of apoptosis for AML cells, and in addition IFNγ seems to inhibit the proliferation of various nonleukaemic cells in the AML microenvironment.
Even though the effects of IFNγ on native human AML cells have been investigated previously [9, 12–18, 32, 33], our recent study [11] describing a possible prognostic impact of IFNγ-induced intracellular signalling in AML cells justifies a more detailed characterization. The previous reports have several limitations. First, they usually include a low number of patients (often less than 10–15), consecutive patients are not examined and the criteria for selection of patients are not given. Secondly, the clinical and biological characteristics of the selected patients are usually incomplete or missing. Finally, only effects on proliferation and membrane molecule expression have been examined, and the experimental models differed. These previous reports have generally described (1) divergent effects of IFNγ on AML colony formation and (2) IFNγ induction of various membrane molecules (i.e. HLA class II molecules, HLA-G, CD54, CD80, CD86, CD95) for subsets of patients. In the present study we therefore investigated the effects of IFNγ for a large group of consecutive and well-characterized patients, and our studies included receptor expression, intracellular signalling events, cytokine release and a detailed characterization of the effects on leukaemia cell proliferation including coculture with stromal cells.
We used well-characterized and standardized experimental models with regard to culture medium and AML cell preparation [21–29]. Our patients represent a consecutive group with high peripheral blood blast counts so that highly enriched AML cells could be prepared without extensive cell separation procedures and thereby the risk of procedure-induced functional alterations [19]. We therefore emphasize that our results may be representative only for this subset of patients, but we avoided further selection by investigating randomly selected or consecutive patient subsets.
We first investigated whether IFNγ could be released when T cells were activated in the presence of proliferating AML cells cocultured in direct contact with normal bone marrow stromal cells. This in vitro model will mimic the natural in vivo microenvironment, and we used an activation signal directed against the TCR/CD3 complex to mimic antigen-specific activation. Our results demonstrated that T cells can be activated to release IFNγ when sharing the microenvironment with AML cells that show constitutive release of several immunoregulatory mediators.
CD119 expression was examined for 39 randomly selected patients, and AML blast expression was detected for all of them and a majority showed strong expression with at least 70% of CD119+ cells. Thus, high expression of CD119 is common and independent of morphology (FAB classification), cytogenetic aberrations or genetic abnormalities of the Flt3 gene. Furthermore, CD119 expression seems to be downregulated after in vitro exposure to IFNγ; this may be due to receptor internalization following receptor ligation. However, the majority of cells are still CD119+ even after the in vitro exposure.
Previous studies have demonstrated that IFNγ can increase AML cells expression of HLA-DR, CD40, CD54, C80, CD95 and possibly CD86 [32, 33]. However, in our cocultures of AML cells and IFNγ secreting T cells only HLA-DR expression was increased whereas the expression of the immunostimulatory molecules CD40 and CD80 and the dendritic cell marker CD83 was not significantly altered. The most likely explanation for the difference between our present and these previous observations [32] is that IFNγ is only a part of a broad T cell cytokine response in our cocultures, and the IFNγ levels reached in our cultures are relatively low compared with the concentrations of exogenous IFNγ used by Costello et al. [32].
The effect of IFNγ ligation on Stat1, Stat3, Stat5 and Erk1/2 phosphorylation status was examined. Increased phosphorylation was observed only for Stat1. CD119 ligation can also induce signalling through Stat1-independent pathways, including Erk1/2-mediated effects [10], but we did not observe any effects on Erk1/2, Stat3 or Stat5 phosphorylation.
IFNγ did not affect spontaneous or IL3- and Flt3L-dependent AML cell proliferation, whereas statistically significant inhibition was observed in the presence of IL1β, SCF, GM-CSF and G-CSF. Both these results and the coculture experiments demonstrated that the growth-inhibitory effect of IFNγ depends on the local cytokine network, and it is not an indirect effect mediated through reduction of IL1β or GM-CSF levels. Finally, IFNγ could also affect AML cell proliferation in the presence of cytarabine and idarubicin, two drugs that are commonly used in AML therapy [3].
We investigated the effects of IFNγ on both stress-induced (spontaneous) and cytarabine-induced AML cell apoptosis, and IFNγ then had divergent effects in both experimental models. Thus, the divergent IFNγ effects represent a true biological variation.
IFNγ caused an increase in the levels of the antiangiogenic chemokines CXCL9–11 and a slight reduction of proangiogenic CXCL8 levels. CXCL9 and CXCL10 expressions are Stat1 dependent, at least in macrophages [10], thus the increased levels are probably caused by the Stat1-mediated signalling. This altered balance between pro- and antiangiogenic signalling may contribute to the inhibition of endothelial cell proliferation in the presence of IFNγ (see below), but it should be emphasized that CXCL8 was still released at relatively high levels even.
We investigated the effects of IFNγ on AML blast proliferation when the cells were cocultured with various nonleukaemic stromal cells. These experiments further demonstrated that the IFNγ effect on blast proliferation was dependent on the local cytokine network. IFNγ inhibited AML blast proliferation significantly only during coculture with microvascular endothelial cells whereas AML cell proliferation was inhibited only for a subset of patients in the presence of fibroblasts, osteoblasts and normal stromal cells. Exogenous IFNγ had an antiproliferative effect on several nonleukaemic cells (i.e. microvascular endothelial cells, osteoblasts, fibroblasts) when added to cocultures of AML blasts and nonleukaemic cells. However, only osteoblastic Cal72 cells and HFL1 fibroblasts but not endothelial cells were directly inhibited by IFNγ when cells were cultured with exogenous IFNγ alone. Taken together our observations therefore suggest that IFNγ can affect the proliferation of nonleukaemic bone marrow stromal cells both directly (fibroblasts, osteoblasts) and indirectly via effects on cocultured AML cells. All these nonleukaemic cells support leukemogenesis [23, 24, 26], and the antiproliferative effects may then indirectly affect the AML cells.
IFNγ can affect the proliferation of various normal cells [40–49], and an important question is then whether IFNγ affects the proliferation of other transformed cells than native human AML cells. AML and ALL are both aggressive malignancies characterized by rapid accumulation of immature and morphologically similar leukaemia blasts in the bone marrow, and for this reason ALL blasts are possibly the most closely related cells to AML blasts. Our results demonstrated that IFNγ could affect the proliferation of different malignant haematopoietic cells, i.e. the effects are not specific for AML blasts.
To conclude, IFNγ can affect AML cell proliferation both when cells are cultured alone and in the presence of nonleukaemic stromal cells. It can also alter the balance between pro- and antiangiogenic chemokine release by the AML cells. These effects may contribute to antileukaemic T cell reactivity after allotransplantation and possibly also in antileukaemic immunotherapy.
Acknowledgement
The work was supported by the Norwegian Cancer Society and Helse-Vest. The technical assistance of Laila Mentzoni and Kristin Paulsen is gratefully acknowledged.
Abbreviations
- AML
Acute myeloid leukaemia
References
- 1.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 2.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Barnett M, Bassan R, Gatta G, Tondini C, Kern W. Adult acute myeloid leukaemia. Crit Rev Oncol Hematol. 2004;50:197–222. doi: 10.1016/j.critrevonc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Bruserud O. Acute myelogenous leukemia blasts as accessory cells during T lymphocyte activation: possible implications for future therapeutic strategies. Leukemia. 1999;13:1175–1187. doi: 10.1038/sj/leu/2401452. [DOI] [PubMed] [Google Scholar]
- 5.Bruserud O, Tjonnfjord G, Gjertsen BT, Foss B, Ernst P. New strategies in the treatment of acute myelogenous leukemia: mobilization and transplantation of autologous peripheral blood stem cells in adult patients. Stem Cells. 2000;18:343–351. doi: 10.1634/stemcells.19-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Bruserud O, Hamann W, Patel S, Ehninger G, Schmidt H, Pawelec G. IFN-gamma and TNF-alpha secretion by CD4+ and CD8+ TCR alpha beta + T-cell clones derived early after allogeneic bone marrow transplantation. Eur J Haematol. 1993;51:73–79. doi: 10.1111/j.1600-0609.1993.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruserud O. Cellular immune responses in acute leukaemia patients with severe chemotherapy-induced leucopenia; characterization of the cytokine repertoire of clonogenic T cells. Cancer Immunol Immunother. 1998;46:221–228. doi: 10.1007/s002620050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone RM, Spriggs DR, Arthur KA, Mayer RJ, Griffin J, Kufe DW. Recombinant human gamma interferon administered by continuous intravenous infusion in acute myelogenous leukemia and myelodysplastic syndromes. Am J Clin Oncol. 1993;16:159–163. doi: 10.1097/00000421-199304000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Beran M, Andersson B, Kantarjian H, Keating M, Rios A, McCredie KB, Freireich EJ, Gutterman J. Hematologic response of four patients with smoldering acute myelogenous leukemia to partially pure gamma interferon. Leukemia. 1987;1:52–57. [PubMed] [Google Scholar]
- 10.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/S1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 11.Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud O, Gjertsen BT, Nolan GP. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–228. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno S, Emi N, Kasai M, Ishitani A, Saito H. Aberrant expression of HLA-G antigen in interferon gamma-stimulated acute myelogenous leukaemia. Br J Haematol. 2000;111:280–282. doi: 10.1046/j.1365-2141.2000.02345.x. [DOI] [PubMed] [Google Scholar]
- 13.Costello RT, Mallet F, Sainty D, Maraninchi D, Gastaut JA, Olive D. Regulation of CD80/B7-1 and CD86/B7-2 molecule expression in human primary acute myeloid leukemia and their role in allogenic immune recognition. Eur J Immunol. 1998;28:90–103. doi: 10.1002/(SICI)1521-4141(199801)28:01<90::AID-IMMU90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Munker R, Andreeff M. Induction of death (CD95/FAS), activation and adhesion (CD54) molecules on blast cells of acute myelogenous leukemias by TNF-alpha and IFN-gamma. Cytokines Mol Ther. 1996;2:147–159. [PubMed] [Google Scholar]
- 15.Nara N. Combined effect of interferon-gamma and tumor necrosis factor-alpha causing suppression of leukemic blast progenitors in acute myeloblastic leukemia. Leuk Lymphoma. 1993;10:201–207. doi: 10.3109/10428199309145884. [DOI] [PubMed] [Google Scholar]
- 16.Murohashi I, Hoang T. Interferon-gamma enhances growth factor-dependent proliferation of clonogenic cells in acute myeloblastic leukemia. Blood. 1991;78:1085–1095. [PubMed] [Google Scholar]
- 17.Kerangueven F, Sempere C, Tabilio A, Mannoni P. Effects of transforming growth factor beta, tumor necrosis factor alpha, interferon gamma and LIF-HILDA on the proliferation of acute myeloid leukemia cells. Eur Cytokine Netw. 1990;1:99–107. [PubMed] [Google Scholar]
- 18.Howell AL, Stukel TA, Bloomfield CD, Davey FR, Ball ED. Induction of differentiation in blast cells and leukemia colony-forming cells from patients with acute myeloid leukemia. Blood. 1990;75:721–729. [PubMed] [Google Scholar]
- 19.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, van’t Veer MB. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9:1783–1786. [PubMed] [Google Scholar]
- 20.Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, Rees JK, Stevens RF, Walker H. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 21.Bruserud O, Hovland R, Wergeland L, Huang TS, Gjertsen BT. Flt3-mediated signaling in human acute myelogenous leukemia (AML) blasts: a functional characterization of Flt3-ligand effects in AML cell populations with and without genetic Flt3 abnormalities. Haematologica. 2003;88:416–428. [PubMed] [Google Scholar]
- 22.Bruserud O, Gjertsen BT, Foss B, Huang TS. New strategies in the treatment of acute myelogenous leukemia (AML): in vitro culture of aml cells—the present use in experimental studies and the possible importance for future therapeutic approaches. Stem Cells. 2001;19:1–11. doi: 10.1634/stemcells.19-1-1. [DOI] [PubMed] [Google Scholar]
- 23.Glenjen N, Ersvaer E, Ryningen A, Bruserud O. In vitro effects of native human acute myelogenous leukemia blasts on fibroblasts and osteoblasts. Int J Cancer. 2004;111:858–867. doi: 10.1002/ijc.20353. [DOI] [PubMed] [Google Scholar]
- 24.Ryningen A, Wergeland L, Glenjen N, Gjertsen BT, Bruserud O. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic interleukin 8. Leuk Res. 2005;29:185–196. doi: 10.1016/j.leukres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Bruserud O, Tronstad KJ, Berge R. In vitro culture of human osteosarcoma cell lines: a comparison of functional characteristics for cell lines cultured in medium without and with fetal calf serum. J Cancer Res Clin Oncol. 2005;131:377–384. doi: 10.1007/s00432-004-0650-z. [DOI] [PubMed] [Google Scholar]
- 26.Rochet N, Dubousset J, Mazeau C, Zanghellini E, Farges MF, de Novion HS, Chompret A, Delpech B, Cattan N, Frenay M, et al. Establishment, characterisation and partial cytokine expression profile of a new human osteosarcoma cell line (CAL 72) Int J Cancer. 1999;82:282–285. doi: 10.1002/(SICI)1097-0215(19990719)82:2<282::AID-IJC20>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Rochet N, Leroy P, Far DF, Ollier L, Loubat A, Rossi B. CAL72: a human osteosarcoma cell line with unique effects on hematopoietic cells. Eur J Haematol. 2003;70:43–52. doi: 10.1034/j.1600-0609.2003.02766.x. [DOI] [PubMed] [Google Scholar]
- 28.Bruserud O, Ryningen A, Wergeland L, Glenjen NI, Gjertsen BT. Osteoblasts increase proliferation and release of pro-angiogenic interleukin 8 by native human acute myelogenous leukemia blasts. Haematologica. 2004;89:391–402. [PubMed] [Google Scholar]
- 29.Bruserud O, Glenjen N, Ryningen A, Ulvestad E. In vitro culture of human acute lymphoblastic leukemia (ALL) cells in serum-free media; a comparison of native ALL blasts, ALL cell lines and virus-transformed B cell lines. Leuk Res. 2003;27:455–464. doi: 10.1016/S0145-2126(02)00227-8. [DOI] [PubMed] [Google Scholar]
- 30.Wendelbo O, Bruserud O. Functional evaluation of proliferative T cell responses in patients with severe T lymphopenia: characterization of optimal culture conditions and standardized activation signals for a simple whole blood assay. J Hematother Stem Cell Res. 2003;12:525–535. doi: 10.1089/152581603322448231. [DOI] [PubMed] [Google Scholar]
- 31.Bruserud O, Mentzoni L, Foss B, Bergheim J, Berentsen S, Nesthus I. Human T lymphocyte activation in the presence of acute myelogenous leukaemia blasts: studies of allostimulated interferon-gamma secretion. Cancer Immunol Immunother. 1996;43:275–282. doi: 10.1007/s002620050334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costello RT, Mallet F, Chambost H, Sainty D, Gastaut JA, Olive D. Differential modulation of immune recognition molecules by interleukin-7 in human acute leukaemias. Eur Cytokine Netw. 1999;10:87–96. [PubMed] [Google Scholar]
- 33.Lecchi M, Lovisone E, Genetta C, Peruccio D, Resegotti L, Richiardi P. Gamma-IFN induces a differential expression of HLA-DR, DQ and DP antigens on peripheral blood myeloid leukemic blasts at various stages of differentiation. Leuk Res. 1989;13:221–226. doi: 10.1016/0145-2126(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 34.Hatfield KJ, Olsnes AM, Gjertsen BT, Bruserud O. Antiangiogenic therapy in acute myelogenous leukemia: targeting of vascular endothelial growth factor and interleukin 8 as possible antileukemic strategies. Curr Cancer Drug Targets. 2005;5:229–248. doi: 10.2174/1568009054064651. [DOI] [PubMed] [Google Scholar]
- 35.Stolze B, Emmendorffer A, Corbacioglu S, Konig A, Welte K, Ebell W. Effects of bone marrow fibroblasts on the proliferation and differentiation of myeloid leukemic cell lines. Exp Hematol. 1995;23:1378–1387. [PubMed] [Google Scholar]
- 36.Bendall LJ, Daniel A, Kortlepel K, Gottlieb DJ. Bone marrow adherent layers inhibit apoptosis of acute myeloid leukemia cells. Exp Hematol. 1994;22:1252–1260. [PubMed] [Google Scholar]
- 37.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5) Exp Hematol. 2001;29:448–457. doi: 10.1016/S0301-472X(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 38.Bruserud O, Ulvestad E. Human acute lymphoblastic leukemia (ALL) blasts as accessory cells during T-cell activation: differences between patients in costimulatory capacity affect proliferative responsiveness and cytokine release by activated T cells. Cancer Immunol Immunother. 2003;52:215–225. doi: 10.1007/s00262-002-0364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruserud O, Wendelboe O. Biological treatment in acute myelogenous leukaemia: how should T-cell targeting immunotherapy be combined with intensive chemotherapy? Expert Opin Biol Ther. 2001;1:1005–1016. doi: 10.1517/14712598.1.6.1005. [DOI] [PubMed] [Google Scholar]
- 40.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–4157. [PubMed] [Google Scholar]
- 41.Kawano Y, Takaue Y, Hirao A, Abe T, Saito S, Matsunaga K, Watanabe T, Hirose M, Ninomiya T, Kuroda Y, et al. Synergistic effect of recombinant interferon-gamma and interleukin-3 on the growth of immature human hematopoietic progenitors. Blood. 1991;77:2118–2121. [PubMed] [Google Scholar]
- 42.Brugger W, Mocklin W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood. 1993;81:2579–2584. [PubMed] [Google Scholar]
- 43.Shiohara M, Koike K, Nakahata T. Synergism of interferon-gamma and stem cell factor on the development of murine hematopoietic progenitors in serum-free culture. Blood. 1993;81:1435–1441. [PubMed] [Google Scholar]
- 44.Tamura T, Ueda S, Yoshida M, Matsuzaki M, Mohri H, Okubo T. Interferon-gamma induces ice gene expression and enhances cellular susceptibility to apoptosis in the U937 leukemia cell line. Biochem Biophys Res Commun. 1996;229:21–26. doi: 10.1006/bbrc.1996.1752. [DOI] [PubMed] [Google Scholar]
- 45.Romagnani S, Giudizi MG, Biagiotti R, Almerigogna F, Mingari C, Maggi E, Liang CM, Moretta L. B cell growth factor activity of interferon-gamma. Recombinant human interferon-gamma promotes proliferation of anti-mu-activated human B lymphocytes. J Immunol. 1986;136:3513–3516. [PubMed] [Google Scholar]
- 46.Grawunder U, Melchers F, Rolink A. Interferon-gamma arrests proliferation and causes apoptosis in stromal cell/interleukin-7-dependent normal murine pre-B cell lines and clones in vitro, but does not induce differentiation to surface immunoglobulin-positive B cells. Eur J Immunol. 1993;23:544–551. doi: 10.1002/eji.1830230237. [DOI] [PubMed] [Google Scholar]
- 47.Novelli F, D’Elios MM, Bernabei P, Ozmen L, Rigamonti L, Almerigogna F, Forni G, Del Prete G. Expression and role in apoptosis of the alpha- and beta-chains of the IFN-gamma receptor on human Th1 and Th2 clones. J Immunol. 1997;159:206–213. [PubMed] [Google Scholar]
- 48.Novelli F, Giovarelli M, Reber-Liske R, Virgallita G, Garotta G, Forni G. Blockade of physiologically secreted IFN-gamma inhibits human T lymphocyte and natural killer cell activation. J Immunol. 1991;147:1445–1452. [PubMed] [Google Scholar]
- 49.Liu Y, Janeway CA., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]