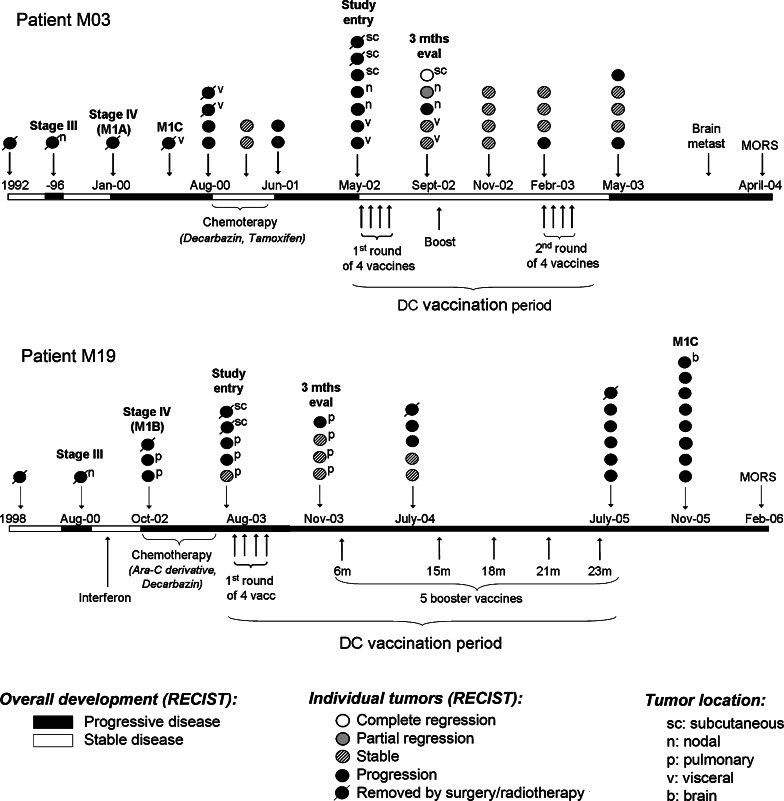
Fig. 1.
Clinical history and DC-vaccination for patients M03 and M19. The overall disease development (RECIST criteria) is indicated by white and black stretches at the horizontal timeline. White stretches represent stable disease, black stretches represent progressive disease. Below the timeline is indicated all systemic therapy, including conventional chemotherapy and DC-vaccination. The circles above the timeline represent individual tumors. The points of disease progression to new AJCC categories are annotated (stage III, stage IV, M1A, M1B, M1C). As indicated, both patients had progressive disease at start of vaccination (RECIST criteria). In patient M03 the evaluation at study entry demonstrated new nodal and subcutaneous lesions, located in the axilles, neck and abdomen. In patient M19 were detected new pulmonary and subcutaneous metastases. The clinical evaluation after 3 months (3 months eval) demonstrated a mixed tumor response in patient M03, as some tumors regressed while other tumors were stable or progressed. In patient M19, the pre-existing pulmonary metastases were stable, but a new pulmonary lesion appeared. Two different DC-vaccines were made for patient M03, based on RNA from different tumor biopsies. The first vaccine was administered intradermally as four weekly injections, and a booster vaccine after 4 months. The second vaccine was administered as four weekly intranodal injections, 9 months after start of vaccination. Patient M19 received all vaccines intradermally and was given five booster injections after the standard four weekly vaccinations