Abstract
CD4+CD25+ regulatory T cells are involved in the prevention of autoimmune diseases and in tumor-induced tolerance. We previously demonstrated in tumor-bearing rodents that one injection of cyclophosphamide could significantly decrease both numbers and suppressive functions of regulatory T cells, facilitating vaccine-induced tumor rejection. In humans, iterative low dosing of cyclophosphamide, referred to as “metronomic” therapy, has recently been used in patients with advanced chemotherapy resistant cancers with the aim of reducing tumor angiogenesis. Here we show that oral administration of metronomic cyclophosphamide in advanced cancer patients induces a profound and selective reduction of circulating regulatory T cells, associated with a suppression of their inhibitory functions on conventional T cells and NK cells leading to a restoration of peripheral T cell proliferation and innate killing activities. Therefore, metronomic regimen of cyclophosphamide does not only affect tumor angiogenesis but also strongly curtails immunosuppressive regulatory T cells, favoring a better control of tumor progression. Altogether these data support cyclophosphamide regimen as a valuable treatment for reducing tumor-induced immune tolerance before setting to work anticancer immunotherapy.
Keywords: Cyclophosphamide, Regulatory T cell, Metronomic treatment, Immunotherapy
Introduction
CD4+CD25+ regulatory T cells (Treg), which constitute about 2-% of CD4+ human T cells [1], are the main contributors to the maintenance of immune tolerance, preventing emergence of organ-specific autoimmune diseases [2]. In tumor-bearing animals, Treg can also curtail antitumor immune responses [3, 4]. In rodents, Treg accumulate in tumor beds and tumor draining lymph nodes, leading to tumor progression [4, 5]. We reported recently that tumor cells could drive immature dendritic cells to express TGF-β required for local expansion of Treg [5]. Human patients suffering from various malignancies also bear increased number of circulating and tumor infiltrating Treg that exert functional inhibition on tumor-specific T cells [6-8]. Treg not only suppress cognate T cell responses but also blunt the innate arm of immunity, inhibiting NK cell proliferation and effector functions through the capacity to down regulate NKG2D level on these cells [9]. NK and T cells inhibition by Treg is associated with a poor prognosis outcome of malignancy in human [8, 9].
In the 1980s, reports claimed that low doses of cyclophosphamide (CTX) could reduce suppressor function of uncharacterized T cells in experimental tumor models [10] and cancer patients [11]. Recently, we demonstrated that one low dose of CTX could dramatically reduce CD4+CD25+ Treg accumulation in tumor bearing animals, restoring the efficiency of immunotherapy against established tumors in rodents [4]. Low dosing of CTX not only decreases the number, but also inhibits the suppressive function of Treg [12, 13]. Such a pharmacological manipulation depleting and/or inhibiting Treg could represent a critical immunointervention in the oncologist’s armamentarium.
It has been recently demonstrated that patients with advanced cancer who developed resistance to chemotherapy could still benefit from iterative injections of distinct cytotoxic drugs at a tenth to a third of the maximum tolerated dose, i.e., a “metronomic-scheduling [14], aimed at inhibiting tumor angiogenesis [15]. Cyclophosphamide is currently used daily as oral low dose in such metronomic scheduling [16, 17], but no immunological follow-up was performed. In this report, we demonstrate that this metronomic treatment selectively targets Treg and restores immune functions in end-stage tumor-bearing patients.
Patients, materials and methods
Following approval by the Regional Ethical Committee for Medical Research (file no. CCPPRB-0509-A2), a sample of 15 ml of peripheral blood was drawn from 9 patients (6 men, 3 women, mean age 52, and range 37-5) with metastatic solid tumors, for which metronomic chemotherapy was indicated after failure of conventional chemotherapy, as well as from 15 age-matched healthy volunteers (HV) (9 men, 6 women, mean age 49, and range 30-2). Patients received CTX 50 mg orally, twice a day 1 week on and 1 week off for 1 month or more. The clinical characteristics of the nine patients are summarized in Table 1.
Table 1.
Patients-characteristics
| Sex | Age | Histology | WHO performance status | Staging | Metastases | Previous treatment | Treatment (duration of CTX) | Steroid | Clinical response |
|---|---|---|---|---|---|---|---|---|---|
| M | 62 | Colon adenocarcinoma | 1 | IV | Lung, liver | FolFox, FolFiri irinotecan Cetuximab | CTX cetuximab (2 months) | Yes | PD |
| M | 65 | Clear cell renal carcinoma | 2 | IV | Peritoneal carcinomatosis | 5FU cisplatin | CTX | No | PD |
| M | 37 | Gastric adenocarcinoma | 1 | IV | Bone, lung, liver | ECF FolFiri | CTX | No | PD |
| F | 48 | Rectal adenocarcinoma | 2 | IV | Peritoneal carcinomatosis, liver, lung | FolFox, FolFiri irinotecan cetuximab | CTX cetuximab (2 months) | No | SD 2 months |
| M | 58 | Pleural mesothelioma | 1 | IV | Lung, mediastinal nodes | Imatinib | CTX imatinib (2 months) | No | SD 3 months |
| F | 61 | Clear cell ovarian cancer | 1 | IV | Peritoneal carcinomatosis | Taxol carboplatin gemcitabin | CTX (2 months) | Yes | SD 3 months |
| M | 37 | Melanoma | 2 | IV | Peritoneal carcinomatosis | Dacarbazin, fortemustine | CTX | No | ND |
| M | 52 | Sarcoma | 1 | IV | Liver | Imatinib | CTX imatinib (2 months) | No | SD 2 months |
| F | 45 | Clear cell renal carcinoma | 1 | IV | Lung, liver | Interferon | CTX | Yes | ND |
PD progressive disease, SD stable disease, ND not determined, M male, F female, CTX cyclophosphamide, 5FU 5 fluorouracil, FolFox 5 FU + oxaliplatin, FolFiri 5FU + irinotecan, ECF epirubicin + cisplatin + 5FU
In the patients and HV, a 15 ml sample of peripheral blood was collected on heparin and used immediately. The phenotype of circulating T and NK cells was studied by whole blood labeling with the following mAbs: fluorescein isothiocyanate-conjugated anti-CD3, phycoerythrin-conjugated anti-CD25 or CD56, allophycocyanin-conjugated anti-CD4 or CD8 (all from Becton Dickinson PharMingen, Heidelberg, Germany) and the corresponding isotype control antibodies. Then the blood samples were treated with the FACS leukocyte fixation and erythrocyte lysis kit (Abcam, Paris, France) and the leukocytes were analyzed by multicolor flow cytometry. The rate of CD4+CD25high T cells, presumably Treg, was determined according to the criteria defined by Baecher-Allan [1]. In four cancer patients, peripheral blood leukocytes were first purified from blood collected before and 1 month after initiating metronomic treatment, using Ficoll/Hypaque (Amersham, Uppsala, Sweden) density centrifugation, then intracellular staining for Foxp3 protein was performed using a Foxp3 kit (http://www.eBioscience.com) according to manufacturer’s instructions. In experiments depleting Treg, PBMC were incubated with anti-CD25 MicroBeads (Miltenyi Biotec, Paris, France) at a concentration of 5 μl/1 × 106 cells for 15 min on ice and washed. The CD4+CD25- fraction was purified over a LD column and expressed more than fourfold less Foxp3 protein than non-depleted cells as assessed by flow cytometry (not shown). To determine NK killing activity, PBMC depleted or not of CD25+ cells were incubated 24 h with CFSE-labeled K562 cells at a 10:1 E:T ratio. K562 cell death was monitored by FACS analysis assessing propidium iodide incorporation in CFSE+ cells. For T cell proliferation assays, CFSE-labeled PBMC (1 μM) depleted or not of CD25+ T cells were cultured with anti-CD3 and anti-CD28 mAbs (Dynal Biotech, Oslo, Norway) according to the manufacturer’s instructions. Proliferation was monitored by FACS analysis assessing the dilution of the CFSE dye at day 5. In some cases, [3H]thymidine (1 μCi per well) was added on day 4 and its incorporation into dividing cells was quantified 18 h later. In five HVs, we had the opportunity to determine the percentage of Treg cells, as well as the NK cell and T cell function on two samples, taken at 1-month interval. We found no significant difference. This establishes the stability of these results in the absence of treatment (not shown data). The error (±) represents standard deviation.
Results
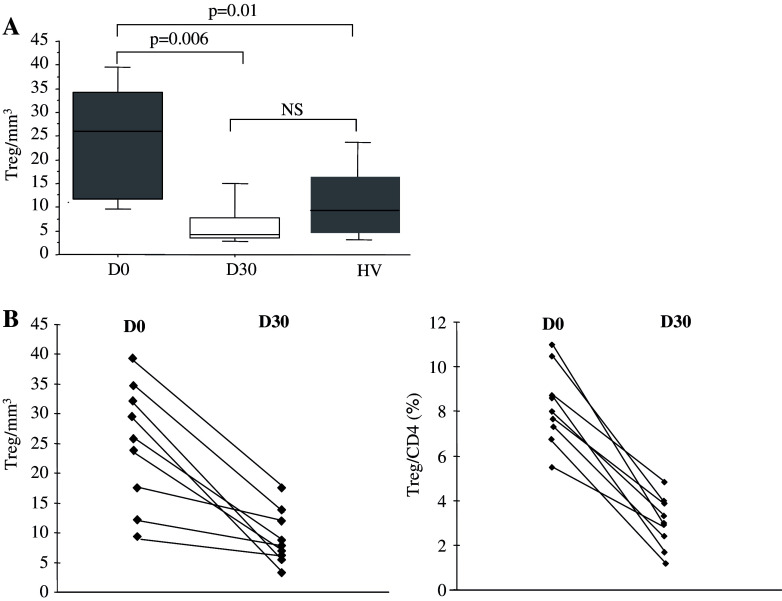
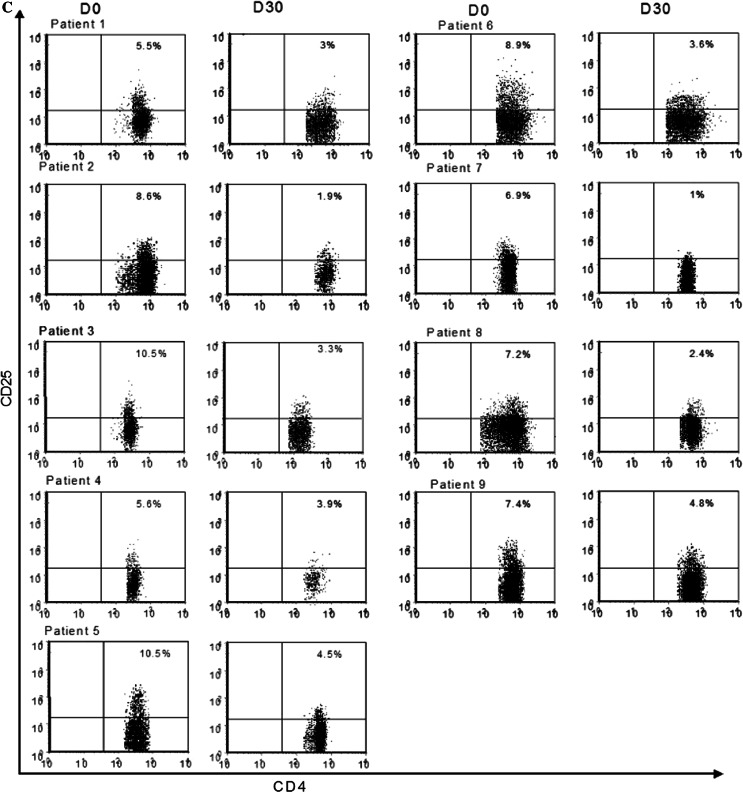
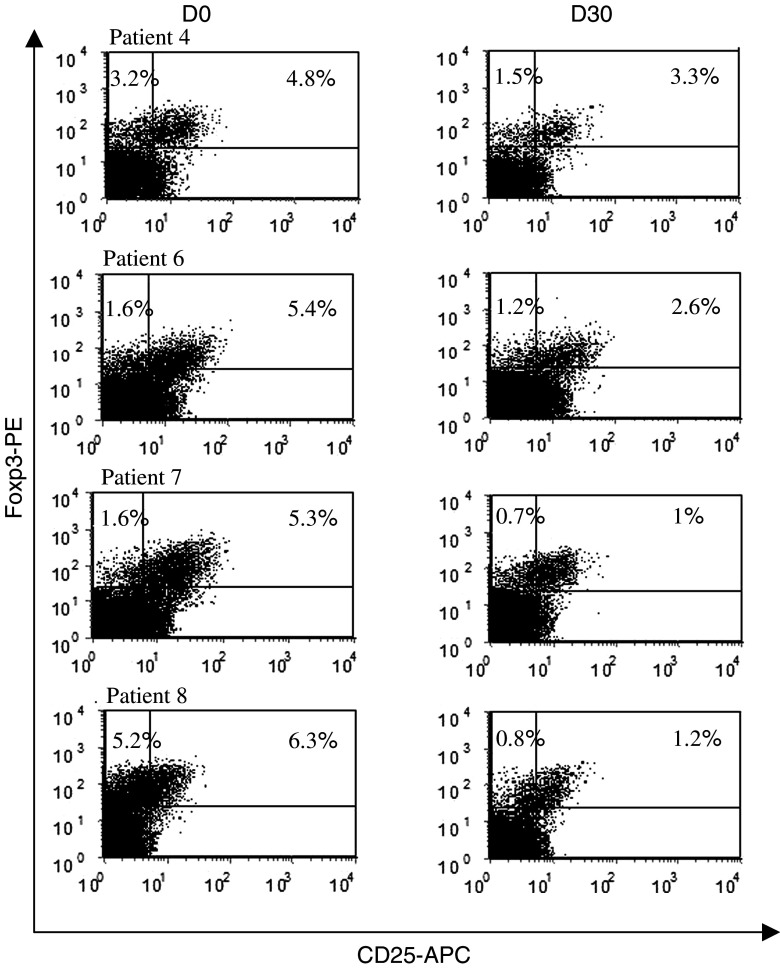
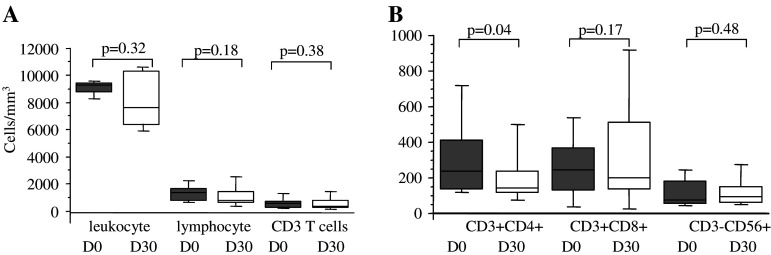
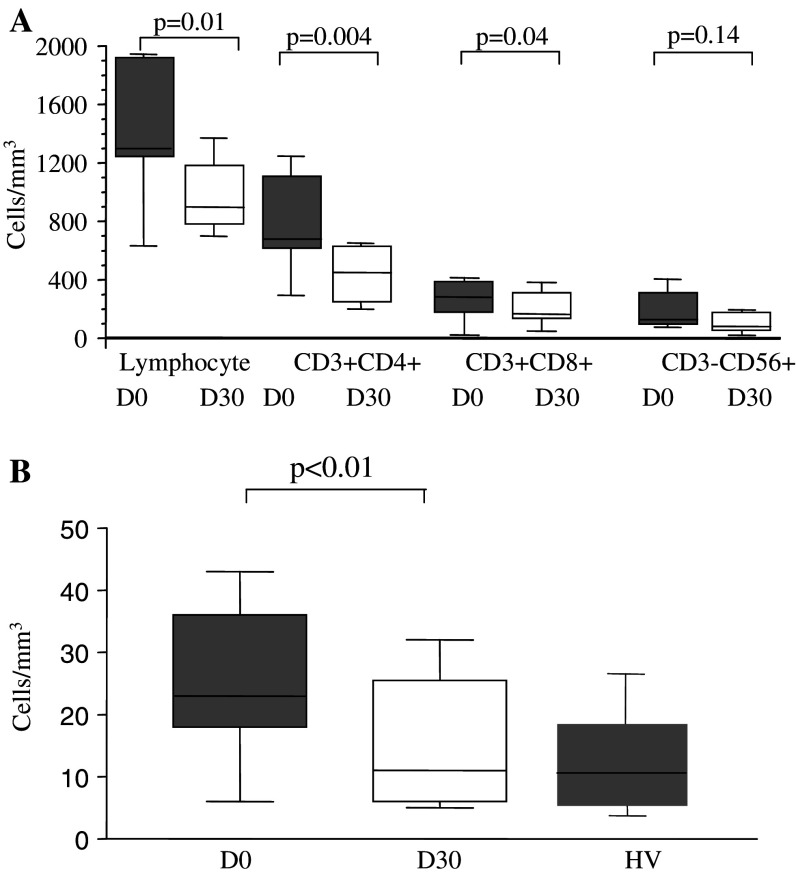
We monitored the effects of the oral metronomic CTX regimen in the nine cancer patients by determining the leukocytes, lymphocytes, CD4+T, CD8+T, NK, and Treg cell subsets in the circulating blood at the start and 1 month after the initiation of therapy. Interestingly, the number of circulating CD4+CD25high Treg in the nine cancer patients bearing end-stage multi-metastatic tumors resistant to conventional therapy was found to be higher before the metronomic treatment than in the HV (Fig. 1a). Moreover, the metronomic CTX treatment induced a dramatic decrease in CD4+CD25high T cells, both in percentages (7.9 ± 1.5% before vs. 3.1 ± 1.8% after, P < 0.0001) and absolute numbers (28.7 ± 9.4 cells/mm3 before vs. 6.4 ± 5.4 cells/mm3 after, P < 0.0001) (Fig. 1a). This decrease in the number and percentage of Treg was observed in each of the nine patients (Fig. 1b, c). We could obtain enough material to determine Foxp3 expression before and after the metronomic treatment in four of these cancer patients and observed a strong decrease in the number of CD25+Foxp3+ cells as well as CD25-Foxp3+ cells during the treatment (Fig. 2). The drop in CD4+CD25high T cell numbers induced by the metronomic treatment was selective since no significant decrease in the number of total leukocytes, as well as T lymphocyte, CD3+ T cell, CD8+ T cell, and CD3-CD56+ NK cell subsets could be observed (Fig. 3a, b). Hence, a 1-month metronomic regimen, with administration of 100 mg CTX per day out of 2 weeks, could selectively deplete the Treg subset in tumor-bearing patients and restore the ranges observed in the HV (Fig. 1a). In two patients, Treg number was measured 2 months after stopping the treatment and found to return to pre-treatment level in both the patients (not shown).
Fig. 1.
Metronomic CTX selectively depletes regulatory T cells (Treg) in cancer patients. a Circulating numbers of CD3+CD4+CD25high per mm3 are determined by FACS analysis. The medians, 75 percentile (box) and SD (whiskers) are depicted for patients (n = 9, Table 1) before (D0) and 1 month after (D30) initiation of metronomic CTX and for healthy volunteers (HV) (n = 15). b Representation of Treg evolution as absolute number (left panel) and percentage of CD4+ T cells (right panel) during metronomic CTX regimen before (D0) and 1 month after (D30) initiation of metronomic CTX for each of the nine treated patients using FACS 3-color staining gating on CD3+CD4+CD25high cells. c Multipanel representation of dot-blot analysis of CD4+CD25high T cells in all patients during metronomic CTX regimen before (D0) and 1 month after (D30) initiation of metronomic CTX for each patients using FACS 3-color staining gating on CD3+CD4+CD25high cells
Fig. 2.
Decrease in the number of Foxp3+ cells during the treatment. Three-color staining gating on CD4+ cells and analysis of CD25 and Foxp3 expression in four patients before and 1 month after initiation of the CTX metronomic treatment
Fig. 3.
Evolution of leukocyte subpopulations during metronomic CTX treatment. a Leukocyte, lymphocyte and CD3+ T lymphocyte counts in the blood of patients (n = 9, Table 1) before and 1 month after initiating metronomic CTX. b Counts of T and NK cell subsets in blood. Flow cytometry analyses allowed to assess the percentages of CD3+CD4+ and CD3+CD8+ lymphocytes and CD3-CD56+ NK cells. Absolute numbers were calculated from whole blood cell enumeration in the nine patients before and 1 month after initiation of metronomic CTX. Statistical analyses used the Fisher’s exact method
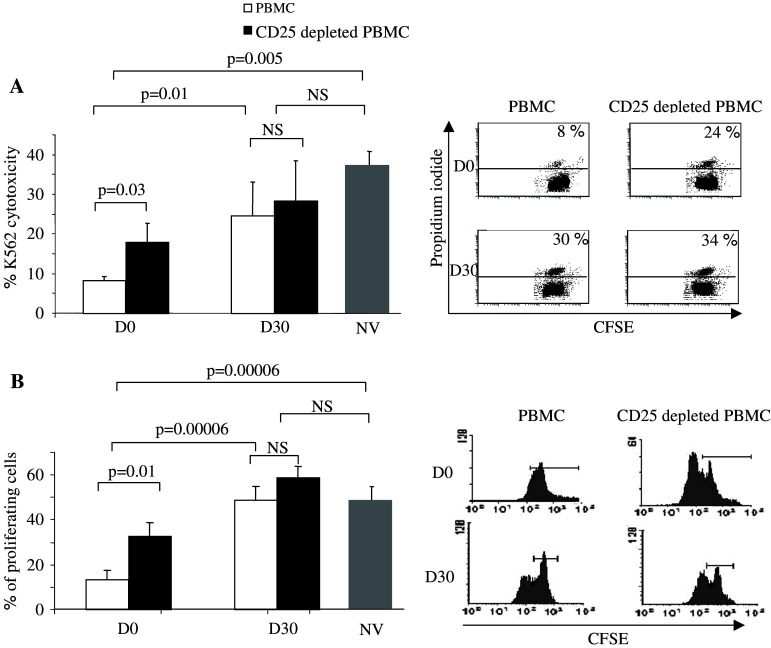
In parallel, we monitored the effects of the metronomic 100 mg per day CTX regimen on the functions of circulating T and NK cells. First, we determined the capacity of blood NK cells to kill NKG2D ligand-expressing K562. While PBMC from five HVs exhibited potent NK cell-dependent cytotoxic activity, PBMC from cancer patients displayed reduced killing capacities before initiation of the metronomic treatment (37 ± 6% vs. 9 ± 1%, respectively, P < 0.005, Fig. 4a). The killing activity of cancer patients-PBMC could be partly restored by depletion of CD25+ cells (Fig. 4a), as expected from our previous report showing that Treg blunted NK cell functions in vitro [9]. More importantly, following 1 month of metronomic CTX treatment, patients-NK cell lytic activity was enhanced and not significantly different from that of the HV (Fig. 4a). It is of note that the residual CD4+CD25high T cells appeared to have lost their NK cell inhibitory activity since ex vivo depletion of CD25+ cells did not improve further the level of cytotoxicity (Fig. 4a). In these patients with advanced tumor, T cell proliferation induced by TCR signaling was dramatically hampered before initiation of the metronomic treatment relatively to the HV (17 ± 3% cells entered in cycle after 5 days of stimulation vs. 45 ± 6% in HV) (P < 0.0001, Fig. 4a), and was partially restored by CD25 depletion in vitro (P = 0.01). One month after initiating CTX treatment, blood T cell proliferation induced by TCR signaling was significantly restored (Fig. 4b) and residual Treg had lost their suppressive function, as ex vivo depletion of CD25+ cells did not improve further TCR-induced T cell proliferation (Fig. 4b).
Fig. 4.
T and NK cell functions are enhanced following metronomic CTX treatment in cancer patients. a Killing of K562. Left panel 1 × 105 PBMC depleted or not from CD25+ cells were mixed with 1 × 104 CFSE-labeled K562 cells for 24 h. K562 cell death was assessed by flow cytometry as the percentage of CFSE labeled cells that incorporated propidium iodide. Graphs depict means ± standard deviation of percentages of dead cells for n = 6 patients (Table 1) before (D0) and after (D30) therapy and for n = 5 healthy volunteers. The right panels show detailed dot-plot analyses for a representative patient at D0 and D30 before or after depletion of CD25+ cells. b Proliferation of T cells induced by TCR signaling. Left panel 1 × 105 CFSE-labeled PBMC depleted or not from CD25+ cells were mixed for 72 h with beads coated with anti-CD3 and anti-CD28 mAbs. Proliferation of T cells was determined by flow cytometry as the percentage of CD3+ cells that diluted the CFSE dye. The graph depicts the mean ± standard deviation of percentages for n = 6 patients at D0 and D30 and for n = 5 healthy volunteers. The right panels show detailed CFSE labeling analyses for a representative patient at D0 and D30 before or after depletion of CD25+ cells. Statistical analyses used the Fisher’s exact method (a, b)
The selective effect of metronomic treatment on the Treg cell population was clearly dependent on the low dosage of CTX. Indeed, in another series of six cancer patients, which received a similar metronomic regimen but with a twice higher dosage of CTX (i.e., 200 mg per day instead of 100 mg), all lymphocytes subpopulations were depleted by the treatment with no specificity on Treg (Fig. 5a, b). This result demonstrates that only low doses of CTX are relevant for obtaining a selective Treg depletion.
Fig. 5.
High dosage CTX (200 mg/day) metronomic treatment looses its specificity in Treg cell depletion. a Lymphocyte, CD3+CD4+ and CD3+CD8+ lymphocytes, and CD3-CD56+ NK cells absolute numbers were determined before and 1 month after initiating high dosage CTX metronomic treatment. b Effect on the CD3+CD4+CD25high Treg. Statistical analyses used the Fisher’s exact method
Discussion
Treg have now been clearly demonstrated to facilitate tumor evasion and to inhibit tumor immune response but no treatment is presently validated to specifically induce Treg depletion in human. A fludarabine-containing regimen was reported to induce Treg depletion in patients bearing chronic lymphocytic leukemia but such a treatment had also a strong impact on other lymphocyte subpopulations and could consequently blunt the effector functions of other immune cells [18]. Administration of a fusion protein combining IL-2 and diphtheria toxin in the aim to destroy regulatory T lymphocytes based on their expression of high-affinity IL-2 receptor failed to eliminate Treg in patients with metastatic melanoma [19].
A preclinical study reported that a metronomic CTX regimen increased the efficacy of a specific tumor vaccine in mice but its effect on Treg was not reported [20]. Here, we demonstrate that metronomic CTX regimen selectively depletes Treg in human while preserving other lymphocyte subsets in number and function. Surprisingly, this metronomic CTX regimen does not inhibit but on the contrary dramatically enhances T and NK cell functions through its suppressive effect on Treg number and function. Thus, a 1 month metronomic CTX regimen, initially designed to reduce tumor angiogenesis through its effect on endothelial cells, also decreases the number of circulating Treg and restores T cell proliferation as well as NK cell effector function in patients bearing end-stage multi-treated tumors. Although CTX clearly depletes Treg in tumor-bearing patients, it may also have additional effects contributing to restoration of the immune response observed after 1 month metronomic treatment. For example, CTX can enhance IFN-α production by splenocytes in a mouse model [21].
Using low dosage of CTX could be a determining factor in the selective effect of this treatment on Treg number and function. Similar trial using higher dose of CTX (200 mg per day) induced a profound decrease in the circulating lymphocyte number, an effect observed on all lymphocyte subpopulations without any selective activity on Treg. Moreover, this treatment decreased the NK cell-dependent cytotoxicity and T cell proliferation capacity (not shown).
These data are arguments suggesting that the protocol described here is worth being tested along with specific immunotherapies aimed at enhancing T cell or NK cell functions in patients bearing advanced cancer.
Acknowledgments
FG received a grant from the Ligue Nationale contre le Cancer (Cote d’Or committee). LZ received a grant from EU ALLOSTEM and DC-THERA and ERM0208 was supported by the Ligue Nationale contre le Cancer.
Footnotes
François Ghiringhelli and Cedric Menard contributed equally to the work.
References
- 1.Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin Immunol. 2005;115:S10–S18. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 3.Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61:8643–8646. [PubMed] [Google Scholar]
- 4.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 5.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 7.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 8.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 9.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- 11.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res. 1987;47:3317–3321. [PubMed] [Google Scholar]
- 12.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 13.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jese G, Lemonnier F, Zitvogel L. Chemo-immunotherapy of tumors: cyclophosphamide synergizes with exosome-based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 14.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–740. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 15.Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003;98:1643–1648. doi: 10.1002/cncr.11713. [DOI] [PubMed] [Google Scholar]
- 17.Shaked Y, Emmenegger U, Francia G, Chen L, Lee CR, Man S, Paraghamian A, Ben-David Y, Kerbel RS. Low dose metronomic combined with intermittent bolus cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–7051. doi: 10.1158/0008-5472.CAN-05-0765. [DOI] [PubMed] [Google Scholar]
- 18.Beyer M, Kochanek M, Darabi K, Endl E, Weihrauch MR, Knolle PA, Classen S, Schultze JL. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukaemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 19.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–8413. [PubMed] [Google Scholar]
- 21.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]