Fig. 3.
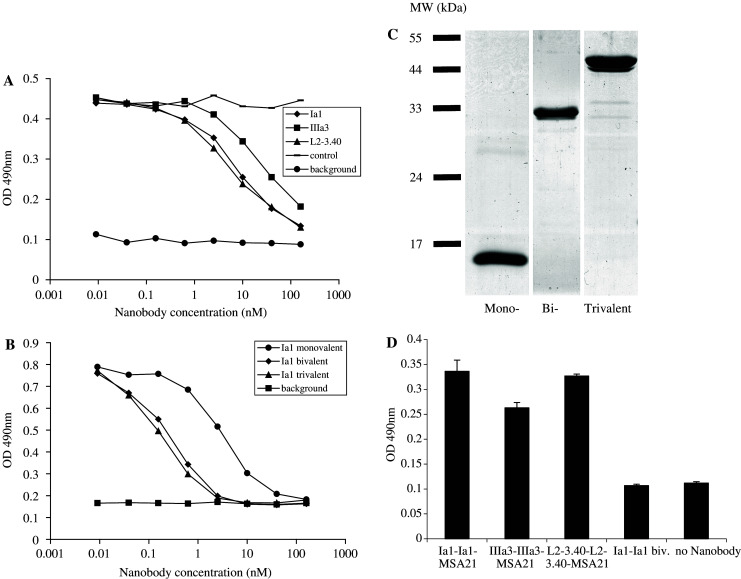
Anti-EGFR Nanobodies compete for EGF binding to the EGFR and trivalent bispecific Nanobodies react simultaneously with EGFR and MSA. a By means of ELISA, the binding of biotinylated EGF to immobilised EGFR was measured in the presence of increasing amounts of a control Nanobody (stripes) or of the anti-EGFR Nanobodies Ia1 (diamonds), IIIa3 (squares) or L2-3.40 (triangles). Background staining was defined as no biotinylated EGF being added (rounds). b Monovalent (rounds), bivalent (diamonds) and trivalent (triangles) variants of anti-EGFR Nanobody Ia1 were tested for their ability to block EGF binding to the EGFR in ELISA. c 3 μg of purified mono-, bi- and trivalent Ia1 Nanobody was size-separated on a 15% poly-acrylamide gel and the gel was stained with coomassie brilliant blue to visualise the proteins. d Trivalent, bispecific Nanobodies (of Ia1, IIIa3 and L2–3.40), or bivalent Ia1 were tested for simultaneous binding to EGFR (coated to the ELISA plate) and biotinylated MSA (used to detect bound Nanobody with alkaline-phosphatase coupled extravidin)
